A multicomponent intervention for the management of chronic pain in older adults: study protocol for a randomized controlled trial
- PMID: 29121961
- PMCID: PMC5680817
- DOI: 10.1186/s13063-017-2270-3
A multicomponent intervention for the management of chronic pain in older adults: study protocol for a randomized controlled trial
Erratum in
-
Correction to: A multicomponent intervention for the management of chronic pain in older adults: study protocol for a randomized controlled trial.Trials. 2021 Nov 25;22(1):842. doi: 10.1186/s13063-021-05780-x. Trials. 2021. PMID: 34823551 Free PMC article. No abstract available.
Abstract
Background: Studies have shown that physical interventions and psychological methods based on the cognitive behavioral approach are efficacious in alleviating pain and that combining both tends to yield more benefits than either intervention alone. In view of the aging population with chronic pain and the lack of evidence-based pain management programs locally, we developed a multicomponent intervention incorporating physical exercise and cognitive behavioral techniques and examined its long-term effects against treatment as usual (i.e., pain education) in older adults with chronic musculoskeletal pain in Hong Kong.
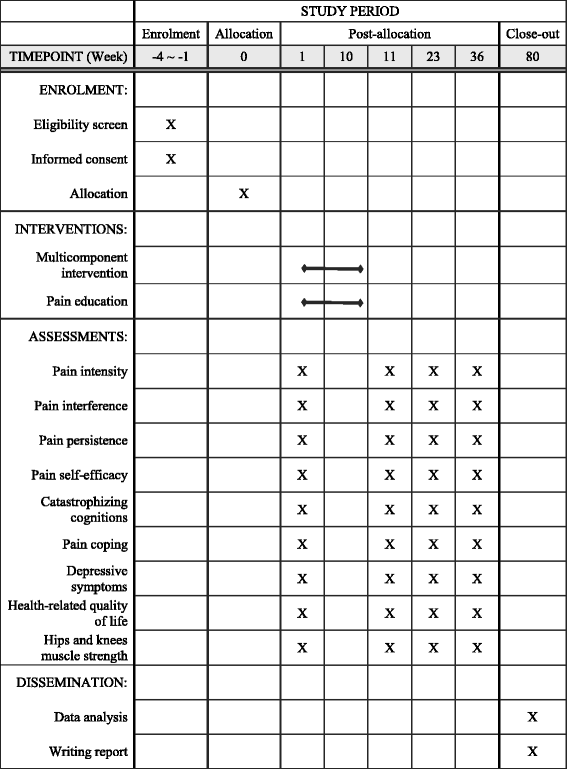
Methods/design: We are conducting a double-blind, cluster-randomized controlled trial. A sample of 160 participants aged ≥ 60 years will be recruited from social centers or outpatient clinics and will be randomized on the basis of center/clinic to either the multicomponent intervention or the pain education program. Both interventions consist of ten weekly sessions of 90 minutes each. The primary outcome is pain intensity, and the secondary outcomes include pain interference, pain persistence, pain self-efficacy, pain coping, pain catastrophizing cognitions, health-related quality of life, depressive symptoms, and hip and knee muscle strength. All outcome measures will be collected at baseline, postintervention, and at 3 and 6 months follow-up. Intention-to-treat analysis will be performed using mixed-effects regression to see whether the multicomponent intervention alleviates pain intensity and associated outcomes over and above the effects of pain education (i.e., a treatment × time intervention effect).
Discussion: Because the activities included in the multicomponent intervention were carefully selected for ready implementation by allied health professionals in general, the results of this study, if positive, will make available an efficacious, nonpharmacological pain management program that can be widely adopted in clinical and social service settings and will hence improve older people's access to pain management services.
Trial registration: Chinese Clinical Trial Registry, ChiCTR-IIR-16008387. Registered on 28 April 2016.
Keywords: Chronic pain management; Cognitive behavioral techniques; Physical exercise.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was provided by The Education University of Hong Kong Human Research Ethics Committee (reference number 2015-2016-0258), the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (reference number 2016.367), the Kowloon Central/Kowloon East Cluster Research Ethics Committee (reference number KC/KE-16-0209/FR-3), and the Kowloon West Cluster Research Ethics Committee [reference number KW/EX-17-004(107-04)]. All participants will be asked to provide written consent to participate.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
An exercise cum cognitive-behavioral intervention for older adults with chronic pain: A cluster-randomized controlled trial.J Consult Clin Psychol. 2022 Mar;90(3):221-233. doi: 10.1037/ccp0000698. Epub 2022 Jan 31. J Consult Clin Psychol. 2022. PMID: 35099206 Clinical Trial.
-
Expectations, effect and experiences of an easily accessible self-management intervention for people with chronic pain: study protocol for a randomised controlled trial with embedded qualitative study.Trials. 2016 Jul 18;17(1):325. doi: 10.1186/s13063-016-1462-6. Trials. 2016. PMID: 27430319 Free PMC article. Clinical Trial.
-
Comparison of a Single-Session Pain Management Skills Intervention With a Single-Session Health Education Intervention and 8 Sessions of Cognitive Behavioral Therapy in Adults With Chronic Low Back Pain: A Randomized Clinical Trial.JAMA Netw Open. 2021 Aug 2;4(8):e2113401. doi: 10.1001/jamanetworkopen.2021.13401. JAMA Netw Open. 2021. PMID: 34398206 Free PMC article. Clinical Trial.
-
Which Interventions Enhance Pain Self-efficacy in People With Chronic Musculoskeletal Pain? A Systematic Review With Meta-analysis of Randomized Controlled Trials, Including Over 12 000 Participants.J Orthop Sports Phys Ther. 2020 Aug;50(8):418-430. doi: 10.2519/jospt.2020.9319. J Orthop Sports Phys Ther. 2020. PMID: 32736497
-
Association Between Psychological Interventions and Chronic Pain Outcomes in Older Adults: A Systematic Review and Meta-analysis.JAMA Intern Med. 2018 Jun 1;178(6):830-839. doi: 10.1001/jamainternmed.2018.0756. JAMA Intern Med. 2018. PMID: 29801109 Free PMC article.
Cited by
-
Educational intervention for promoting stretching exercise behavior among a sample of Iranian office employees: applying the Health Promotion Model.J Pain Res. 2019 Feb 22;12:733-742. doi: 10.2147/JPR.S183410. eCollection 2019. J Pain Res. 2019. PMID: 30863146 Free PMC article.
-
Lower education is an associated factor with the combination of pain catastrophizing and kinesiophobia in patients with knee osteoarthritis?Clin Rheumatol. 2021 Jun;40(6):2361-2367. doi: 10.1007/s10067-020-05518-1. Epub 2020 Nov 23. Clin Rheumatol. 2021. PMID: 33230685
-
eHealth-Integrated Psychosocial and Physical Interventions for Chronic Pain in Older Adults: Scoping Review.J Med Internet Res. 2024 Jul 29;26:e55366. doi: 10.2196/55366. J Med Internet Res. 2024. PMID: 39073865 Free PMC article.
-
Correction to: A multicomponent intervention for the management of chronic pain in older adults: study protocol for a randomized controlled trial.Trials. 2021 Nov 25;22(1):842. doi: 10.1186/s13063-021-05780-x. Trials. 2021. PMID: 34823551 Free PMC article. No abstract available.
-
Mapping review of pain management programmes and psychological therapies for community-dwelling older people living with pain.Eur Geriatr Med. 2024 Feb;15(1):33-45. doi: 10.1007/s41999-023-00871-1. Epub 2023 Oct 18. Eur Geriatr Med. 2024. PMID: 37853269 Free PMC article. Review.
References
-
- Jones GT, Macfarlane GA. Epidemiology of pain in older persons. In: Gibson SJ, Weiner DK, editors. Pain in older persons. Seattle, WA: IASP Press; 2005. pp. 3–24.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous