A randomised, double-blind, placebo-controlled trial of minocycline and/or omega-3 fatty acids added to treatment as usual for at-risk mental states (NAYAB): study protocol
- PMID: 29121974
- PMCID: PMC5679379
- DOI: 10.1186/s13063-017-2275-y
A randomised, double-blind, placebo-controlled trial of minocycline and/or omega-3 fatty acids added to treatment as usual for at-risk mental states (NAYAB): study protocol
Abstract
Background: The at-risk mental state (ARMS) describes individuals at high risk of developing schizophrenia or psychosis. The use of antipsychotics in this population is not supported, because most individuals with ARMS are unlikely to develop psychosis. Anti-inflammatory treatments and polyunsaturated fatty acids (PUFAs) may have some beneficial effects in the treatment of ARMS. There have been no controlled clinical trials in which researchers have investigated the use of minocycline for ARMS and no trials involving PUFAs in combination with other proposed treatments. There is a need to find effective, tolerable and inexpensive interventions for individuals with ARMS that are available in high-, low- and middle-income countries.
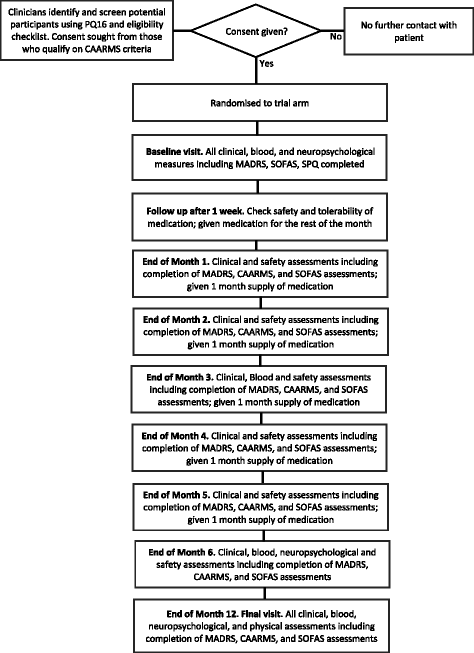
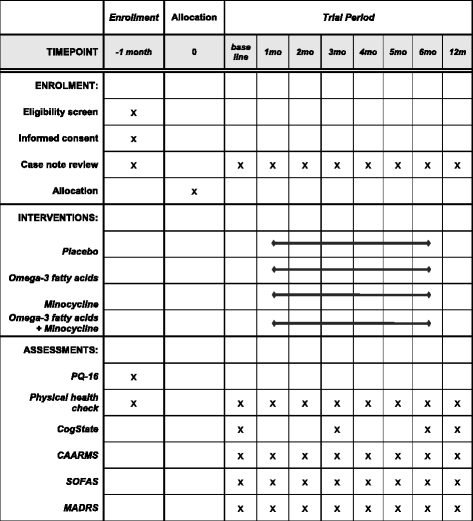
Methods/design: A 6-month intervention study of minocycline and/or omega-3 fatty acids added to treatment as usual (TAU) in patients with ARMS will be conducted in Pakistan using a randomised, placebo-controlled, double-blind factorial design. A total of 320 consenting patients with capacity will be recruited from the community, general practitioner clinics and psychiatric units. Allowing for a 25% dropout rate, we will recruit 59 completing participants into each study arm, and in total 236 will complete the study. We will determine whether the addition of minocycline and/or omega-3 fatty acids to TAU attenuates the rate of transition from ARMS to first-episode psychosis and improves symptoms and/or level of functioning in ARMS. We will also investigate whether any candidate risk factors, such as negative symptoms, influence treatment response in the ARMS group. The primary efficacy endpoint is conversion to psychotic disorder at 12 months after study entry. Analysis will be done according to the intention to treat principle using analysis of variance, chi-square tests and adjusted ORs to assess between-group differences. Cox regression analysis will be used to evaluate potential between-group differences in time to onset of psychosis.
Discussion: The outcomes of this trial will provide evidence of the potential benefits of minocycline and PUFAs in the treatment of ARMS. Both minocycline and PUFAs are inexpensive, are readily available in low-/middle-income countries such as Pakistan, and if proven, may be safe and effective for treating individuals with ARMS.
Trial registration: ClinicalTrials.gov, NCT02569307 . Registered on 3 October 2015.
Keywords: At-risk mental state (ARMS); Global mental health; Minocycline; Omega-3 fatty acids; PUFA; Ultra-high risk (UHR) schizophrenia.
Conflict of interest statement
Ethics approval and consent to participate
Institutional review board (IRB) approval has been obtained from the ethics committee of the Karachi Medical and Dental College and Dow University of Health Sciences, Pakistan. Written consent will be obtained from each participant prior to any trial-related data collection.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

