The Pocket-4-Life project, bioavailability and beneficial properties of the bioactive compounds of espresso coffee and cocoa-based confectionery containing coffee: study protocol for a randomized cross-over trial
- PMID: 29121975
- PMCID: PMC5680745
- DOI: 10.1186/s13063-017-2271-2
The Pocket-4-Life project, bioavailability and beneficial properties of the bioactive compounds of espresso coffee and cocoa-based confectionery containing coffee: study protocol for a randomized cross-over trial
Abstract
Background: Coffee is an important source of bioactive compounds, including caffeine, phenolic compounds (mainly chlorogenic acids), trigonelline, and diterpenes. Several studies have highlighted the preventive effects of coffee consumption on major cardiometabolic diseases, but the impact of coffee dosage on markers of cardiometabolic risk is not well understood. Moreover, the pool of coffee-derived circulating metabolites and the contribution of each metabolite to disease prevention still need to be evaluated in real-life settings. The aim of this study will be to define the bioavailability and beneficial properties of coffee bioactive compounds on the basis of different levels of consumption, by using an innovative experimental design. The contribution of cocoa-based products containing coffee to the pool of circulating metabolites and their putative bioactivity will also be investigated.
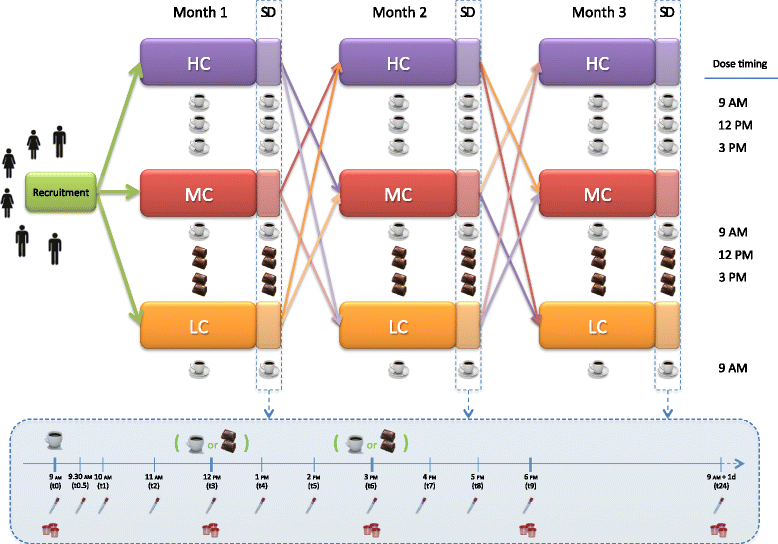
Methods: A three-arm, crossover, randomized trial will be conducted. Twenty-one volunteers will be randomly assigned to consume three treatments in a random order for 1 month: 1 cup of espresso coffee/day, 3 cups of espresso coffee/day, and 1 cup of espresso coffee plus 2 cocoa-based products containing coffee twice per day. The last day of each treatment, blood and urine samples will be collected at specific time points, up to 24 hours following the consumption of the first product. At the end of each treatment the same protocol will be repeated, switching the allocation group. Besides the bioavailability of the coffee/cocoa bioactive compounds, the effect of the coffee/cocoa consumption on several cardiometabolic risk factors (anthropometric measures, blood pressure, inflammatory markers, trimethylamine N-oxide, nitric oxide, blood lipids, fasting indices of glucose/insulin metabolism, DNA damage, eicosanoids, and nutri-metabolomics) will be investigated.
Discussion: Results will provide information on the bioavailability of the main groups of phytochemicals in coffee and on their modulation by the level of consumption. Findings will also show the circulating metabolites and their bioactivity when coffee consumption is substituted with the intake of cocoa-based products containing coffee. Finally, the effect of different levels of 1-month coffee consumption on cardiometabolic risk factors will be elucidated, likely providing additional insights on the role of coffee in the protection against chronic diseases.
Trial registration: ClinicalTrials.gov, NCT03166540 . Registered on May 21, 2017.
Keywords: Bioavailability; Caffeine; Caffeoylquinic acid; Cardiometabolic risk factors; Cocoa; Coffee; Diterpenes; Flavan-3-ols; Pharmacokinetic; Trigonelline.
Conflict of interest statement
Ethics approval and consent to participate
The Ethics Committee of the University of Parma approved the study on October 12, 2016 (AZOSPR/0036174/6.2.2.). The protocol was modified to include further analysis and the ethical approval for these amendments was granted on April 19, 2017 (AZOSPR/0015693/6.2.2.). All trial participants will provide full written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Effect of coffee and cocoa-based confectionery containing coffee on markers of cardiometabolic health: results from the pocket-4-life project.Eur J Nutr. 2021 Apr;60(3):1453-1463. doi: 10.1007/s00394-020-02347-5. Epub 2020 Jul 29. Eur J Nutr. 2021. PMID: 32728879 Free PMC article. Clinical Trial.
-
Effect of Coffee and Cocoa-Based Confectionery Containing Coffee on Markers of DNA Damage and Lipid Peroxidation Products: Results from a Human Intervention Study.Nutrients. 2021 Jul 13;13(7):2399. doi: 10.3390/nu13072399. Nutrients. 2021. PMID: 34371907 Free PMC article. Clinical Trial.
-
Effect of different patterns of consumption of coffee and a cocoa-based product containing coffee on the nutrikinetics and urinary excretion of phenolic compounds.Am J Clin Nutr. 2021 Dec 1;114(6):2107-2118. doi: 10.1093/ajcn/nqab299. Am J Clin Nutr. 2021. PMID: 34582552 Clinical Trial.
-
Flavan-3-ols, theobromine, and the effects of cocoa and chocolate on cardiometabolic risk factors.Curr Opin Lipidol. 2015 Feb;26(1):10-9. doi: 10.1097/MOL.0000000000000144. Curr Opin Lipidol. 2015. PMID: 25551798 Review.
-
Drinking for protection? Epidemiological and experimental evidence on the beneficial effects of coffee or major coffee compounds against gastrointestinal and liver carcinogenesis.Food Res Int. 2019 Sep;123:567-589. doi: 10.1016/j.foodres.2019.05.029. Epub 2019 May 22. Food Res Int. 2019. PMID: 31285007 Review.
Cited by
-
Pomegranate juice to reduce fecal calprotectin levels in inflammatory bowel disease patients with a high risk of clinical relapse: Study protocol for a randomized controlled trial.Trials. 2019 Jun 6;20(1):327. doi: 10.1186/s13063-019-3321-8. Trials. 2019. PMID: 31171016 Free PMC article.
-
Data analysis of MS-based clinical lipidomics studies with crossover design: A tutorial mini-review of statistical methods.Clin Mass Spectrom. 2019 May 20;13:5-17. doi: 10.1016/j.clinms.2019.05.002. eCollection 2019 Aug. Clin Mass Spectrom. 2019. PMID: 34841080 Free PMC article. Review.
-
Effects of the Consumption of Low-Fat Cooked Ham with Reduced Salt Enriched with Antioxidants on the Improvement of Cardiovascular Health: A Randomized Clinical Trial.Nutrients. 2021 Apr 27;13(5):1480. doi: 10.3390/nu13051480. Nutrients. 2021. PMID: 33925704 Free PMC article. Clinical Trial.
-
Effect of coffee and cocoa-based confectionery containing coffee on markers of cardiometabolic health: results from the pocket-4-life project.Eur J Nutr. 2021 Apr;60(3):1453-1463. doi: 10.1007/s00394-020-02347-5. Epub 2020 Jul 29. Eur J Nutr. 2021. PMID: 32728879 Free PMC article. Clinical Trial.
-
Effect of Polyphenol-Rich Interventions on Gut Microbiota and Inflammatory or Oxidative Stress Markers in Adults Who Are Overweight or Obese: A Systematic Review and Meta-Analysis.Nutrients. 2025 Jul 29;17(15):2468. doi: 10.3390/nu17152468. Nutrients. 2025. PMID: 40806053 Free PMC article. Review.
References
-
- Tresserra-Rimbau A, Rimm EB, Medina-Remón A, Martínez-González MA, de la Torre R, Corella D, et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr Metab Cardiovasc Dis. 2014;24:639–47. doi: 10.1016/j.numecd.2013.12.014. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

