Prevalence of tick-borne pathogens in questing Ixodes ricinus ticks in urban and suburban areas of Switzerland
- PMID: 29121976
- PMCID: PMC5680829
- DOI: 10.1186/s13071-017-2500-2
Prevalence of tick-borne pathogens in questing Ixodes ricinus ticks in urban and suburban areas of Switzerland
Abstract
Background: Throughout Europe, Ixodes ricinus transmits numerous pathogens. Its widespread distribution is not limited to rural but also includes urbanized areas. To date, comprehensive data on pathogen carrier rates of I. ricinus ticks in urban areas of Switzerland is lacking.
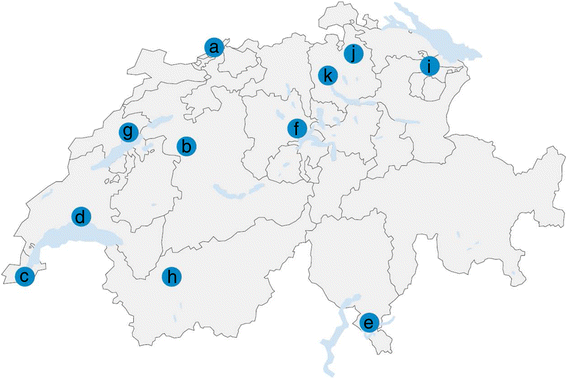
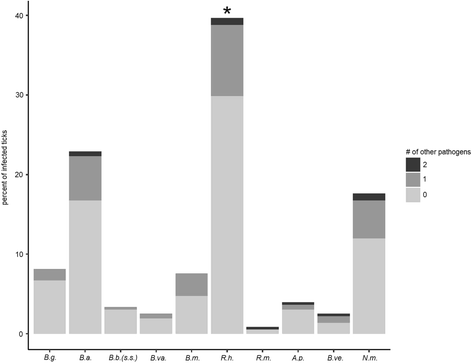
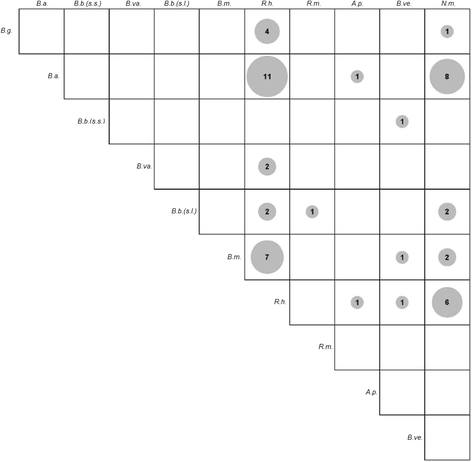
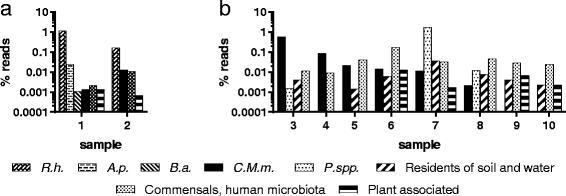
Results: Ixodes ricinus ticks sampled at 18 (sub-) urban collection sites throughout Switzerland showed carrier rates of 0% for tick-borne encephalitis virus, 18.0% for Borrelia burgdorferi (sensu lato), 2.5% for Borrelia miyamotoi, 13.5% for Rickettsia spp., 1.4% for Anaplasma phagocytophilum, 6.2% for "Candidatus Neoehrlichia mikurensis", and 0.8% for Babesia venatorum (Babesia sp., EU1). Site-specific prevalence at collection sites with n > 45 ticks (n = 9) significantly differed for B. burgdorferi (s.l.), Rickettsia spp., and "Ca. N. mikurensis", but were not related to the habitat type. Three hundred fifty eight out of 1078 I. ricinus ticks (33.2%) tested positive for at least one pathogen. Thereof, about 20% (71/358) were carrying two or three different potentially disease-causing agents. Using next generation sequencing, we could detect true pathogens, tick symbionts and organisms of environmental or human origin in ten selected samples.
Conclusions: Our data document the presence of pathogens in the (sub-) urban I. ricinus tick population in Switzerland, with carrier rates as high as those in rural regions. Carriage of multiple pathogens was repeatedly observed, demonstrating the risk of acquiring multiple infections as a consequence of a tick bite.
Keywords: "Candidatus Midichloria mitochondrii"; "Candidatus Neoehrlichia mikurensis"; Anaplasma; Babesia; Borrelia; Ixodes ricinus; NGS; Rickettsia; Tick-borne encephalitis virus; Urban.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Gray JS. Biology of Ixodes species ticks in relation to tick-borne zoonoses. Wien Klin Wochenschr. 2002;114(13-14):473–478. - PubMed
-
- Ecker M, Allison SL, Meixner T, Heinz FX. Sequence analysis and genetic classification of tick-borne encephalitis viruses from Europe and Asia. J Gen Virol. 1999;80(Pt 1):179–185. - PubMed
-
- Lindquist L. Tick-borne encephalitis. In: Tselis ACB, editor. Handbook of Clinical Neurology, vol. 123, 1 edn. Amsterdam, Netherlands: Elsevier; 2014. pp. 531–559. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

