Platelet inhibition during ticagrelor monotherapy versus ticagrelor plus aspirin in patients with coronary artery disease (TEMPLATE study): study protocol for a randomised controlled trial
- PMID: 29121979
- PMCID: PMC5680755
- DOI: 10.1186/s13063-017-2277-9
Platelet inhibition during ticagrelor monotherapy versus ticagrelor plus aspirin in patients with coronary artery disease (TEMPLATE study): study protocol for a randomised controlled trial
Abstract
Background: Dual antiplatelet therapy (DAPT) with aspirin (ASP) and a P2Y12 blocker is currently standard care after percutaneous coronary intervention (PCI) with stent insertion, and aims to inhibit platelet function in order to prevent stent thrombosis. The P2Y12 blocker ticagrelor (TIC) has greater antiplatelet effect than the previously used members of this class, such as clopidogrel. In healthy volunteers, TIC is sufficient to cause strong platelet inhibition, with little additional effect from ASP. Omission of ASP may improve the safety of antiplatelet regimes by reducing bleeding. However, the effect of single antiplatelet treatment with TIC, compared to DAPT with TIC + ASP, has not been studied in detail in patients with coronary artery disease.
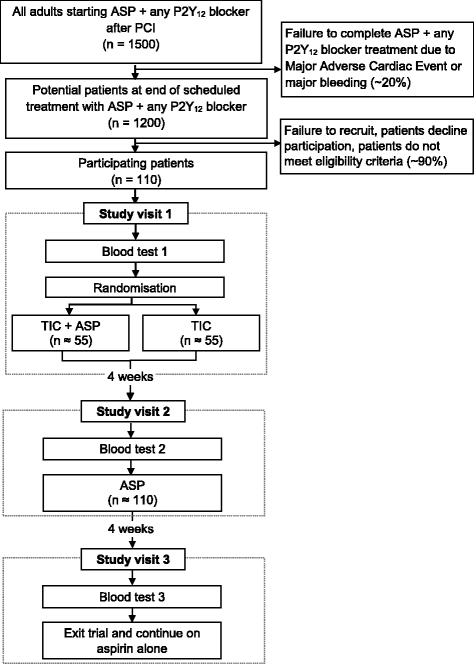
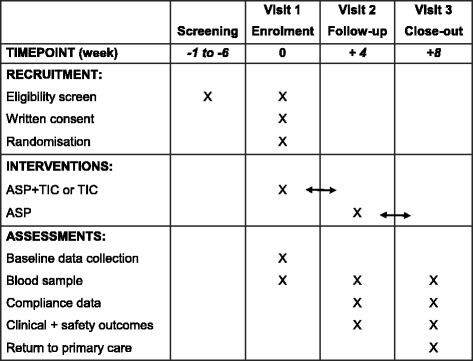
Methods: To compare TIC with TIC + ASP, we have initiated a single centre, open-label randomised controlled trial (TEMPLATE study) in adults receiving DAPT following PCI with a sample size of 110 patients. Patients are invited to join the study when, as part of standard care, they are due to switch from DAPT (ASP + any P2Y12 blocker) to single antiplatelet treatment with ASP alone after 6-12 months. Patients are randomised to receive either TIC or TIC + ASP for 4 weeks. All patients then revert to standard care with ASP alone. Blood samples and clinical data are collected at three study visits: at baseline during treatment with ASP + any P2Y12 blocker (visit 1); approximately 4 weeks after visit 1 during treatment with either TIC or TIC + ASP (visit 2); and approximately 8 weeks after visit 1 when treatment has reverted to ASP alone (visit 3). The primary outcome is the extent of platelet inhibition, measured by light transmission aggregation, flow cytometry, flow chamber and plasma biomarker tests. The primary analysis will compare the extent of platelet inhibition between the TIC and TIC + ASP groups at visit 2, adjusted for baseline platelet reactivity. Secondary analyses will compare the extent of platelet inhibition at visit 2 with that at visit 3.
Discussion: This is the first study to compare in detail the extent of platelet inhibition in patients who are receiving TIC compared with TIC + ASP. The study findings will complement larger-scale trials of the clinical efficacy and safety of TIC compared to TIC + ASP.
Trial registration: ISRCTN registry, identifier ISRCTN84335288 . Registered on 23 June 2014.
Keywords: Antiplatelet therapy; Aspirin; Clopidogrel; Haematology; P2Y12 blocker; Platelet function; Prasugrel; Ticagrelor.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
Research ethics approval was granted by the South-Central Oxford A Research Ethics Committee (reference 14/SC/1309). Written informed consent is required from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Pharmacodynamic Comparison of Ticagrelor Monotherapy Versus Ticagrelor and Aspirin in Patients After Percutaneous Coronary Intervention: The TEMPLATE (Ticagrelor Monotherapy and Platelet Reactivity) Randomized Controlled Trial.J Am Heart Assoc. 2020 Dec 15;9(24):e016495. doi: 10.1161/JAHA.120.016495. Epub 2020 Dec 11. J Am Heart Assoc. 2020. PMID: 33305660 Free PMC article. Clinical Trial.
-
Serum uric acid levels during dual antiplatelet therapy with ticagrelor or clopidogrel: Results from a single-centre study.Nutr Metab Cardiovasc Dis. 2016 Jul;26(7):567-574. doi: 10.1016/j.numecd.2016.03.001. Epub 2016 Mar 15. Nutr Metab Cardiovasc Dis. 2016. PMID: 27134063
-
Ticagrelor alone versus ticagrelor plus aspirin from month 1 to month 12 after percutaneous coronary intervention in patients with acute coronary syndromes (ULTIMATE-DAPT): a randomised, placebo-controlled, double-blind clinical trial.Lancet. 2024 May 11;403(10439):1866-1878. doi: 10.1016/S0140-6736(24)00473-2. Epub 2024 Apr 7. Lancet. 2024. PMID: 38599220 Clinical Trial.
-
Platelet adenosine diphosphate receptor antagonists: ticlopidine to ticagrelor-a long continuing journey.Indian Heart J. 2012 Jan-Feb;64(1):54-9. doi: 10.1016/S0019-4832(12)60012-1. Epub 2012 Mar 26. Indian Heart J. 2012. PMID: 22572427 Free PMC article. Review.
-
Long-term antiplatelet therapy following myocardial infarction: implications of PEGASUS-TIMI 54.Heart. 2016 May 15;102(10):783-9. doi: 10.1136/heartjnl-2015-307858. Epub 2016 Feb 8. Heart. 2016. PMID: 26857211 Review.
Cited by
-
Pharmacodynamic Comparison of Ticagrelor Monotherapy Versus Ticagrelor and Aspirin in Patients After Percutaneous Coronary Intervention: The TEMPLATE (Ticagrelor Monotherapy and Platelet Reactivity) Randomized Controlled Trial.J Am Heart Assoc. 2020 Dec 15;9(24):e016495. doi: 10.1161/JAHA.120.016495. Epub 2020 Dec 11. J Am Heart Assoc. 2020. PMID: 33305660 Free PMC article. Clinical Trial.
-
P2Y12 Inhibitor Monotherapy versus Conventional Dual Antiplatelet Therapy in Patients with Acute Coronary Syndrome after Percutaneous Coronary Intervention: A Meta-Analysis.Pharmaceuticals (Basel). 2023 Feb 3;16(2):232. doi: 10.3390/ph16020232. Pharmaceuticals (Basel). 2023. PMID: 37259380 Free PMC article.
-
Efficacy and safety of ticagrelor monotherapy following a brief DAPT vs. prolonged 12-month DAPT in ACS patients post-PCI: a meta-analysis of RCTs.Eur J Clin Pharmacol. 2024 Dec;80(12):1871-1882. doi: 10.1007/s00228-024-03747-w. Epub 2024 Sep 12. Eur J Clin Pharmacol. 2024. PMID: 39264445
References
-
- Anderson JL, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426–579. doi: 10.1161/CIR.0b013e318212bb8b. - DOI - PubMed
-
- Sibbing D, et al. Response to letter regarding article, “Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement”. Circulation. 2010;122(14):e479. doi: 10.1161/CIRCULATIONAHA.110.967158. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous