Production and characterization of monoclonal antibodies against Encephalitozoon intestinalis and Encephalitozoon sp. spores and their developmental stages
- PMID: 29121996
- PMCID: PMC5680761
- DOI: 10.1186/s13071-017-2503-z
Production and characterization of monoclonal antibodies against Encephalitozoon intestinalis and Encephalitozoon sp. spores and their developmental stages
Abstract
Background: Microsporidia are intracellular obligate parasites traditionally associated with immunosuppressed patients; their detection in immunocompetent patients has increased, highlighting their possible importance as emerging pathogens. Detection of spores in stools, urine, body fluids and tissues is difficult and immunological techniques such as immunofluorescence have proved to be a useful and reliable tool in the diagnosis of human microsporidiosis. For this reason, we have produced and characterized monoclonal antibodies (MAbs) specific for Encephalitozoon intestinalis (the second most frequent microsporidian infecting humans), and other Encephalitozoon species, that can be used in different diagnostic techniques.
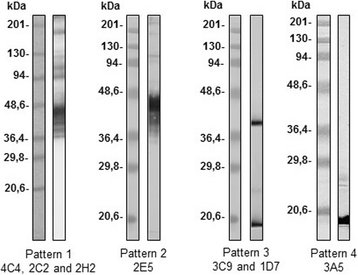
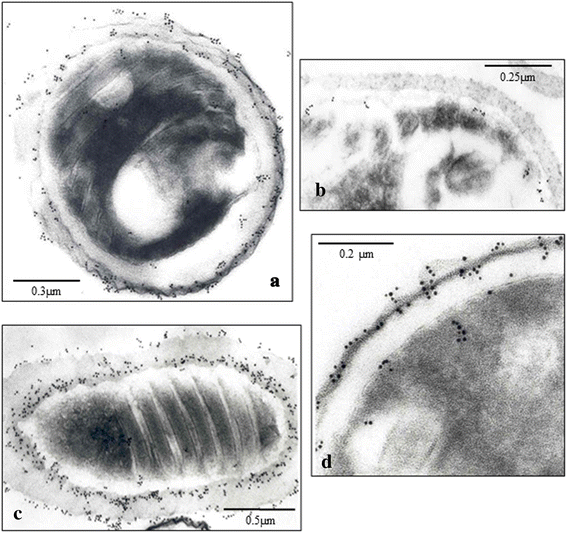
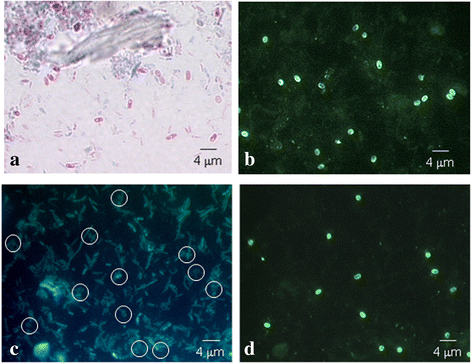
Results: Seven MAbs were selected in accordance with their optical density (OD). Four (4C4, 2C2, 2E5 and 2H2) were isotype IgG2a; two (3A5 and 3C9) isotype IgG3, and one Mab, 1D7, IgM isotype. The selected monoclonal antibody-secreting hybridomas were characterized by indirect immunofluorescence antibody test (IFAT), enzyme-linked immunosorbent assay (ELISA), Western blot, immunoelectron microscopy (Immunogold) and in vitro cultures. The study by IFAT showed different behavior depending on the MAbs studied. The MAbs 4C4, 2C2, 2E5 and 2H2 showed reactivity against epitopes in the wall of the spore (exospore and endospore) epitopes located in Encephalitozoon sp. spores, whereas the MAbs 3A5, 1D7 and 3C9 showed reactivity against internal epitopes (cytoplasmic contents or sporoplasm) of E. intestinalis spores. All MAbs recognized the developing parasites in the in vitro cultures of E. intestinalis. Additionally, 59 formalin-fixed stool samples that had been previously analyzed were screened, with 26 (44%) presenting microsporidian spores (18 samples with E. intestinalis and 8 samples with Enterocytozoon bieneusi). Detection of microsporidian spores by microscopy was performed using Calcofluor stain, Modified Trichrome, Quick-Hot Gram Chromotrope, as well as IFAT using MAbs 4C4, 2C2, 2E5 and 2H2. The 4 MAbs tested clearly recognized the larger spores corresponding to E. intestinalis, but showed no reactivity with Enterocytozoon bieneusi spores. The mass spectrometry and proteomic study revealed that the Mabs 4C4, 2C2, 2E5 and 2H2 recognized the Spore Wall Protein 1 (SWP1) as the antigenic target.
Conclusions: The IFAT-positive MAbs exhibited excellent reactivity against spores and developmental stages, permitting their use in human and animal diagnosis. The epitopes recognized (exospore, endospore and cytoplasmic contents) by the different MAbs developed need further study, and may reveal potential targets for vaccine development, immunotherapy and chemotherapy.
Keywords: Developmental stages; Diagnosis; Encephalitozoon intestinalis; Encephalitozoon sp.; Monoclonal antibodies; Spores.
Conflict of interest statement
Ethics approval and consent to participate
Human fecal samples: all fecal samples were from patients in Atlanta hospitals and established in the CDC fecal bank at the Division of Parasitic Diseases (“Measure the incidence of and risk factors of diarrhea associated with parasitic infections among people infected with HIV” from January 1, 1991 to September 30, 1994/CDC). Informed consent was obtained from patients, and the guidelines for human experimentation of the U.S. Department of Health and Human Services (HHS) were followed. All samples were analyzed anonymously. The authors confirm that the study was reviewed and approved by an institutional review board (HHS) following the Basic HHS Policy for Protection of Human Research Subjects before the study began. Animals: all of the experimental procedures were carried out in accordance with Spanish legislation (Real Decreto 223/1988) and European Union (Council Directive 86/609/EEC) on the use of experimental animals, use of infectious agents and laboratory safety (Real Decreto 664/1997). The studies and protocols were approved by the Veterinary Commission of the University San Pablo CEU (references no. 09/98 and 01/99).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Retrospective species identification of microsporidian spores in diarrheic fecal samples from human immunodeficiency virus/AIDS patients by multiplexed fluorescence in situ hybridization.J Clin Microbiol. 2007 Apr;45(4):1255-60. doi: 10.1128/JCM.01975-06. Epub 2007 Feb 7. J Clin Microbiol. 2007. PMID: 17287331 Free PMC article.
-
[Prevalence of Encephalitozoon intestinalis and Enterocytozoon bieneusi in cancer patients under chemotherapy].Mikrobiyol Bul. 2015 Jan;49(1):105-13. doi: 10.5578/mb.8787. Mikrobiyol Bul. 2015. PMID: 25706736 Turkish.
-
Simple diagnosis of Encephalitozoon sp. microsporidial infections by using a panspecific antiexospore monoclonal antibody.J Clin Microbiol. 1997 Mar;35(3):724-9. doi: 10.1128/jcm.35.3.724-729.1997. J Clin Microbiol. 1997. PMID: 9041420 Free PMC article.
-
Intestinal microsporidiosis.Semin Gastrointest Dis. 1997 Jan;8(1):45-55. Semin Gastrointest Dis. 1997. PMID: 9000501 Review.
-
Microsporidial AIDS cholangiopathy due to Encephalitozoon intestinalis: case report and review.Am J Gastroenterol. 2000 Sep;95(9):2364-71. doi: 10.1111/j.1572-0241.2000.02334.x. Am J Gastroenterol. 2000. PMID: 11007244 Review.
Cited by
-
Latent Microsporidia Infection Prevalence as a Risk Factor in Colon Cancer Patients.Cancers (Basel). 2022 Oct 29;14(21):5342. doi: 10.3390/cancers14215342. Cancers (Basel). 2022. PMID: 36358760 Free PMC article.
-
Microsporidia in Rodents-Mus musculus, Rattus norvegicus, and Rattus rattus-A Public Health Concern in the Canary Islands, Spain.Animals (Basel). 2025 Jun 8;15(12):1695. doi: 10.3390/ani15121695. Animals (Basel). 2025. PMID: 40564247 Free PMC article.
-
Comparison of histologic methods for the detection of Desmozoon lepeophtherii spores in the gills of Atlantic salmon.J Vet Diagn Invest. 2020 Jan;32(1):142-146. doi: 10.1177/1040638719887707. Epub 2019 Nov 18. J Vet Diagn Invest. 2020. PMID: 31735129 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

