d-Leucine: Evaluation in an epilepsy model
- PMID: 29122492
- PMCID: PMC5756680
- DOI: 10.1016/j.yebeh.2017.09.003
d-Leucine: Evaluation in an epilepsy model
Abstract
Background: Current medicines do not provide sufficient seizure control for nearly one-third of patients with epilepsy. New options are needed to address this treatment gap. We recently found that the atypical amino acid d-leucine protected against acutely-induced seizures in mice, but its effect in chronic seizures has not been explored. We hypothesized that d-leucine would protect against spontaneous recurrent seizures. We also investigated whether mice lacking a previously-described d-leucine receptor (Tas1R2/R3) would be protected against acutely-induced seizures.
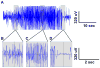
Methods: Male FVB/NJ mice were subjected to kainic acid-induced status epilepticus and monitored by video-electroencephalography (EEG) (surgically implanted electrodes) for 4weeks before, during, and after treatment with d-leucine. Tas1R2/R3 knockout mice and controls underwent the maximal electroshock threshold (MES-T) and 6-Hz tests.
Results: There was no difference in number of calendar days with seizures or seizure frequency with d-leucine treatment. In an exploratory analysis, mice treated with d-leucine had a lower number of dark cycles with seizures. Tas1R2/R3 knockout mice had elevated seizure thresholds in the MES-T test but not the 6-Hz test.
Conclusions: d-Leucine treatment was ineffective against chronic seizures after kainic acid-induced status epilepticus, but there was some efficacy during the dark cycle. Because d-leucine is highly concentrated in the pineal gland, these data suggest that d-leucine may be useful as a tool for studying circadian patterns in epilepsy. Deletion of the Tas1R2/R3 receptor protected against seizures in the MES-T test and, therefore, may be a novel target for treating seizures.
Keywords: Epilepsy; Kainic acid; Sleep; Taste receptors; d-Amino acid.
Published by Elsevier Inc.
Figures

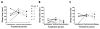

References
-
- Kobau R, Zahran H, Thurman DJ, Zack MM, Henry TR, Schachter SC, et al. Epilepsy surveillance among adults--19 States, Behavioral Risk Factor Surveillance System, 2005. MMWR Surveill Summ. 2008;57:1–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

