CRISPR-mediated deletion of the PECAM-1 cytoplasmic domain increases receptor lateral mobility and strengthens endothelial cell junctional integrity
- PMID: 29122551
- PMCID: PMC5754039
- DOI: 10.1016/j.lfs.2017.11.002
CRISPR-mediated deletion of the PECAM-1 cytoplasmic domain increases receptor lateral mobility and strengthens endothelial cell junctional integrity
Abstract
Aims: PECAM-1 is an abundant endothelial cell surface receptor that becomes highly enriched at endothelial cell-cell junctions, where it functions to mediate leukocyte transendothelial migration, sense changes in shear and flow, and maintain the vascular permeability barrier. Homophilic interactions mediated by the PECAM-1 extracellular domain are known to be required for PECAM-1 to perform these functions; however, much less is understood about the role of its cytoplasmic domain in these processes.
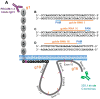
Main methods: CRISPR/Cas9 gene editing technology was employed to generate human endothelial cell lines that either lack PECAM-1 entirely, or express mutated PECAM-1 missing the majority of its cytoplasmic domain (∆CD-PECAM-1). The endothelial barrier function was evaluated by Electric Cell-substrate Impedance Sensing, and molecular mobility was assessed by fluorescence recovery after photobleaching.
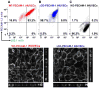
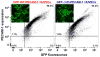
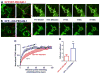
Key findings: We found that ∆CD-PECAM-1 concentrates normally at endothelial cell junctions, but has the unexpected property of conferring increased baseline barrier resistance, as well as a more rapid rate of recovery of vascular integrity following thrombin-induced disruption of the endothelial barrier. Fluorescence recovery after photobleaching analysis revealed that ∆CD-PECAM-1 exhibits increased mobility within the plane of the plasma membrane, thus allowing it to redistribute more rapidly back to endothelial cell-cell borders to reform the vascular permeability barrier.
Significance: The PECAM-1 cytoplasmic domain plays a novel role in regulating the rate and extent of vascular permeability following thrombotic or inflammatory challenge.
Keywords: Adhesion; Endothelial cell; Glycosylation; PECAM-1; Permeability; Sialic acid; Vascular biology.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures

References
-
- Bergom C, Goel R, Paddock C, Gao C, Newman DK, Matsuyama S, et al. The cell-adhesion and signaling molecule PECAM-1 is a molecular mediator of resistance to genotoxic chemotherapy. Cancer Biol Ther. 2006;5:1699–707. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

