Mitral Valve Prolapse: Multimodality Imaging and Genetic Insights
- PMID: 29122631
- PMCID: PMC5805573
- DOI: 10.1016/j.pcad.2017.10.007
Mitral Valve Prolapse: Multimodality Imaging and Genetic Insights
Abstract
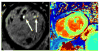
Mitral valve prolapse (MVP) is a common heritable valvulopathy affecting approximately 2.4% of the population. It is the most important cause of primary mitral regurgitation (MR) requiring surgery. MVP is characterized by fibromyxomatous changes and displacement of one or both mitral leaflets into the left atrium. Echocardiography represents the primary diagnostic modality for assessment of MVP. Accurate quantitation of ventricular volumes and function for surgical planning in asymptomatic severe MR can be provided with both echocardiography and cardiac magnetic resonance. In addition, assessment of myocardial fibrosis using late gadolinium enhancement and T1 mapping allows better understanding of the impact of MVP on the myocardium. Imaging in MVP is important not only for diagnostic and prognostic purposes, but is also essential for detailed phenotyping in genetic studies. Genotype-phenotype studies in MVP pedigrees have allowed the identification of milder, non-diagnostic MVP morphologies by echocardiography. Such morphologies represent early expression of MVP in gene carriers. This review focuses on multimodality imaging and the phenotypic spectrum of MVP. Moreover, the review details the recent genetic discoveries that have increased our understanding of the pathophysiology of MVP, with clues to mechanisms and therapy.
Keywords: Cardiac magnetic resonance; Echocardiography; Genetics; Mitral regurgitation; Mitral valve prolapse.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest: None.
Figures


References
-
- Freed LA, Levy D, Levine RA, Larson MG, Evans JC, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7. - PubMed
-
- Zuppiroli A, Rinaldi M, Kramer-Fox R, Favilli S, Roman MJ, Devereux RB. Natural history of mitral valve prolapse. Am J Cardiol. 1995;75:1028–32. - PubMed
-
- Devereux RB, Kramer-Fox R, Shear MK, Kligfield P, Pini R, Savage DD. Diagnosis and classification of severity of mitral valve prolapse: methodologic, biologic, and prognostic considerations. Am Heart J. 1987;113:1265–80. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

