Myotube migration to cover and shape the testis of Drosophila depends on Heartless, Cadherin/Catenin, and myosin II
- PMID: 29122742
- PMCID: PMC5769643
- DOI: 10.1242/bio.025940
Myotube migration to cover and shape the testis of Drosophila depends on Heartless, Cadherin/Catenin, and myosin II
Abstract
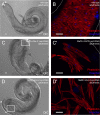
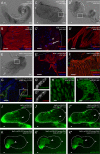
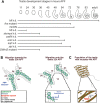
During Drosophila metamorphosis, nascent testis myotubes migrate from the prospective seminal vesicle of the genital disc onto pupal testes and then further to cover the testes with multinucleated smooth-like muscles. Here we show that DWnt2 is likely required for determination of testis-relevant myoblasts on the genital disc. Knock down of fibroblast growth factor receptor (FGFR) heartless by RNAi and a dominant-negative version revealed multiple functions of Heartless, namely regulation of the amount of myoblasts on the genital disc, connection of seminal vesicles and testes, and migration of muscles along the testes. Live imaging indicated that the downstream effector Stumps is required for migration of testis myotubes on the testis towards the apical tip. After myoblast fusion, myosin II is needed for migration of nascent testis myotubes, in which Thisbe-dependent fibroblast growth factor (FGF) signaling is activated. Cadherin-N is essential for connecting these single myofibers and for creating a firm testis muscle sheath that shapes and stabilizes the testis tubule. Based on these results, we propose a model for the migration of testis myotubes in which nascent testis myotubes migrate as a collective onto and along the testis, dependent on FGF-regulated expression of myosin II.
Keywords: DWnt2; FGF; Muscles; Stumps; Testes tubules; Thisbe.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures





Similar articles
-
A new level of plasticity: Drosophila smooth-like testes muscles compensate failure of myoblast fusion.Development. 2016 Jan 15;143(2):329-38. doi: 10.1242/dev.126730. Epub 2015 Dec 10. Development. 2016. PMID: 26657767 Free PMC article.
-
mir-276a Is Required for Muscle Development in Drosophila and Regulates the FGF Receptor Heartless During the Migration of Nascent Myotubes in the Testis.Cells. 2025 Mar 3;14(5):368. doi: 10.3390/cells14050368. Cells. 2025. PMID: 40072096 Free PMC article.
-
A large reverse-genetic screen identifies numerous regulators of testis nascent myotube collective cell migration and collective organ sculpting.Mol Biol Cell. 2025 Feb 1;36(2):ar21. doi: 10.1091/mbc.E24-10-0456. Epub 2025 Jan 2. Mol Biol Cell. 2025. PMID: 39745864 Free PMC article.
-
Collective cell migration driven by filopodia-New insights from the social behavior of myotubes.Bioessays. 2021 Nov;43(11):e2100124. doi: 10.1002/bies.202100124. Epub 2021 Sep 4. Bioessays. 2021. PMID: 34480489 Review.
-
FGFs and their receptors, in vitro and in vivo studies: new FGF receptor in the brain, FGF-1 in muscle, and the use of functional analogues of low-affinity heparin-binding growth factor receptors in tissue repair.Mol Reprod Dev. 1994 Sep;39(1):49-54; discussion 54-5. doi: 10.1002/mrd.1080390109. Mol Reprod Dev. 1994. PMID: 7528027 Review.
Cited by
-
Reproductive isolation caused by azoospermia in sterile male hybrids of Drosophila.Ecol Evol. 2020 May 4;10(12):5922-5931. doi: 10.1002/ece3.6329. eCollection 2020 Jun. Ecol Evol. 2020. PMID: 32607201 Free PMC article.
-
Reproduction disrupts stem cell homeostasis in testes of aged male Drosophila via an induced microenvironment.PLoS Genet. 2019 Jul 11;15(7):e1008062. doi: 10.1371/journal.pgen.1008062. eCollection 2019 Jul. PLoS Genet. 2019. PMID: 31295251 Free PMC article.
-
Drosophila hamlet mediates epithelial tissue assembly of the reproductive system.Elife. 2025 Jul 4;13:RP104164. doi: 10.7554/eLife.104164. Elife. 2025. PMID: 40613556 Free PMC article.
-
The RNF220 domain nuclear factor Teyrha-Meyrha (Tey) regulates the migration and differentiation of specific visceral and somatic muscles in Drosophila.Development. 2023 Sep 15;150(18):dev201457. doi: 10.1242/dev.201457. Epub 2023 Sep 14. Development. 2023. PMID: 37642089 Free PMC article.
-
Filopodia-based contact stimulation of cell migration drives tissue morphogenesis.Nat Commun. 2021 Feb 4;12(1):791. doi: 10.1038/s41467-020-20362-2. Nat Commun. 2021. PMID: 33542237 Free PMC article.
References
-
- Baylies M. K., Martinez Arias A. and Bate M. (1995). wingless is required for the formation of a subset of muscle founder cells during Drosophila embryogenesis. Development 121, 3829-3837. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

