Frontline defenders: goblet cell mediators dictate host-microbe interactions in the intestinal tract during health and disease
- PMID: 29122749
- PMCID: PMC5899238
- DOI: 10.1152/ajpgi.00181.2017
Frontline defenders: goblet cell mediators dictate host-microbe interactions in the intestinal tract during health and disease
Abstract
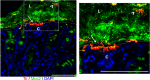
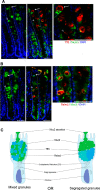
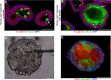
Goblet cells (GCs) are the predominant secretory epithelial cells lining the luminal surface of the mammalian gastrointestinal (GI) tract. Best known for their apical release of mucin 2 (Muc2), which is critical for the formation of the intestinal mucus barrier, GCs have often been overlooked for their active contributions to intestinal protection and host defense. In part, this oversight reflects the limited tools available to study their function but also because GCs have long been viewed as relatively passive players in promoting intestinal homeostasis and host defense. In light of recent studies, this perspective has shifted, as current evidence suggests that Muc2 as well as other GC mediators are actively released into the lumen to defend the host when the GI tract is challenged by noxious stimuli. The ability of GCs to sense and respond to danger signals, such as bacterial pathogens, has recently been linked to inflammasome signaling, potentially intrinsic to the GCs themselves. Moreover, further work suggests that GCs release Muc2, as well as other mediators, to modulate the composition of the gut microbiome, leading to both the expansion as well as the depletion of specific gut microbes. This review will focus on the mechanisms by which GCs actively defend the host from noxious stimuli, as well as describe advanced technologies and new approaches by which their responses can be addressed. Taken together, we will highlight current insights into this understudied, yet critical, aspect of intestinal mucosal protection and its role in promoting gut defense and homeostasis.
Keywords: goblet cell; gut infections; inflammatory bowel disease; microbes; mucus.
Figures

Similar articles
-
The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression.Int J Biol Macromol. 2020 Dec 1;164:884-891. doi: 10.1016/j.ijbiomac.2020.07.191. Epub 2020 Jul 22. Int J Biol Macromol. 2020. PMID: 32707285 Review.
-
Bifidobacterium dentium Fortifies the Intestinal Mucus Layer via Autophagy and Calcium Signaling Pathways.mBio. 2019 Jun 18;10(3):e01087-19. doi: 10.1128/mBio.01087-19. mBio. 2019. PMID: 31213556 Free PMC article.
-
Entamoeba histolytica-Induced Mucin Exocytosis Is Mediated by VAMP8 and Is Critical in Mucosal Innate Host Defense.mBio. 2017 Oct 3;8(5):e01323-17. doi: 10.1128/mBio.01323-17. mBio. 2017. PMID: 28974617 Free PMC article.
-
The Nlrp6 inflammasome is not required for baseline colonic inner mucus layer formation or function.J Exp Med. 2019 Nov 4;216(11):2602-2618. doi: 10.1084/jem.20190679. Epub 2019 Aug 16. J Exp Med. 2019. PMID: 31420376 Free PMC article.
-
Intestinal goblet cells and mucins in health and disease: recent insights and progress.Curr Gastroenterol Rep. 2010 Oct;12(5):319-30. doi: 10.1007/s11894-010-0131-2. Curr Gastroenterol Rep. 2010. PMID: 20703838 Free PMC article. Review.
Cited by
-
Polyphenols-Gut-Heart: An Impactful Relationship to Improve Cardiovascular Diseases.Antioxidants (Basel). 2022 Aug 30;11(9):1700. doi: 10.3390/antiox11091700. Antioxidants (Basel). 2022. PMID: 36139775 Free PMC article. Review.
-
The Protective Role of Scorias spongiosa Polysaccharide-Based Microcapsules on Intestinal Barrier Integrity in DSS-Induced Colitis in Mice.Foods. 2023 Feb 3;12(3):669. doi: 10.3390/foods12030669. Foods. 2023. PMID: 36766197 Free PMC article.
-
Human Milk Oligosaccharide Supplementation Affects Intestinal Barrier Function and Microbial Composition in the Gastrointestinal Tract of Young Sprague Dawley Rats.Nutrients. 2020 May 25;12(5):1532. doi: 10.3390/nu12051532. Nutrients. 2020. PMID: 32466125 Free PMC article.
-
Beneficial Effect of Mildly Pasteurized Whey Protein on Intestinal Integrity and Innate Defense in Preterm and Near-Term Piglets.Nutrients. 2020 Apr 17;12(4):1125. doi: 10.3390/nu12041125. Nutrients. 2020. PMID: 32316586 Free PMC article.
-
Targeting the intestinal circadian clock by meal timing ameliorates gastrointestinal inflammation.Cell Mol Immunol. 2024 Aug;21(8):842-855. doi: 10.1038/s41423-024-01189-z. Epub 2024 Jun 25. Cell Mol Immunol. 2024. PMID: 38918576 Free PMC article.
References
-
- Alipour M, Zaidi D, Valcheva R, Jovel J, Martínez I, Sergi C, Walter J, Mason AL, Wong GK, Dieleman LA, Carroll MW, Huynh HQ, Wine E. Mucosal barrier depletion and loss of bacterial diversity are primary abnormalities in paediatric ulcerative colitis. J Crohn’s Colitis 10: 462–471, 2016. doi: 10.1093/ecco-jcc/jjv223. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

