Soluble CD80 Protein Delays Tumor Growth and Promotes Tumor-Infiltrating Lymphocytes
- PMID: 29122838
- PMCID: PMC7262951
- DOI: 10.1158/2326-6066.CIR-17-0026
Soluble CD80 Protein Delays Tumor Growth and Promotes Tumor-Infiltrating Lymphocytes
Abstract
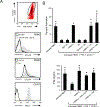
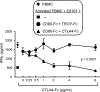
Tumor cells use various immune-suppressive strategies to overcome antitumor immunity. One such method is tumor expression of programmed death ligand-1 (PD-L1), which triggers apoptotic death or anergy upon binding programmed death-1 (PD-1) on T cells. Our previous in vitro cellular studies with human and mouse PD-L1+ tumor cells demonstrated that a soluble form of the costimulatory molecule CD80 prevented PD-L1-mediated immune suppression and restored T-cell activation by binding PD-L1 and blocking interaction with PD-1. We now report that in vivo treatment of established syngeneic PD-L1+ CT26 colon carcinoma and B16F10 melanoma tumors with CD80-Fc delays tumor growth and promotes tumor-infiltrating T cells. Studies with PD-1-/- and CD28-/- mice demonstrate that soluble CD80 acts in vivo by simultaneously neutralizing PD-1 suppression and activating through CD28. We also report that soluble CD80 mediates its effects by activating transcription factors EGR1-4, NF-κB, and MAPK, downstream signaling components of the CD28 and T-cell receptor pathways. Soluble CD80 binds to CTLA-4 on activated human peripheral blood mononuclear cells. However, increasing quantities of CTLA-4 antagonist antibodies do not increase T-cell activation. These results indicate that soluble CD80 does not suppress T-cell function through CTLA-4 and suggest that CTLA-4 acts as a decoy receptor for CD80, rather than functioning as a suppressive signaling receptor. Collectively, these studies demonstrate that soluble CD80 has therapeutic efficacy in vivo in mouse tumor systems and that its effects are due to its ability to inhibit PD-1-mediated suppression while concurrently activating T cells through CD28. Cancer Immunol Res; 6(1); 59-68. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest statement: The authors declare no conflicts of interest
Figures

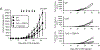


Similar articles
-
A soluble form of CD80 enhances antitumor immunity by neutralizing programmed death ligand-1 and simultaneously providing costimulation.Cancer Immunol Res. 2014 Jul;2(7):610-5. doi: 10.1158/2326-6066.CIR-13-0204. Epub 2014 Apr 2. Cancer Immunol Res. 2014. PMID: 24819296 Free PMC article.
-
PD-L1:CD80 Cis-Heterodimer Triggers the Co-stimulatory Receptor CD28 While Repressing the Inhibitory PD-1 and CTLA-4 Pathways.Immunity. 2019 Dec 17;51(6):1059-1073.e9. doi: 10.1016/j.immuni.2019.11.003. Epub 2019 Nov 19. Immunity. 2019. PMID: 31757674 Free PMC article.
-
By Binding CD80 and CD86, the Vaccinia Virus M2 Protein Blocks Their Interactions with both CD28 and CTLA4 and Potentiates CD80 Binding to PD-L1.J Virol. 2019 May 15;93(11):e00207-19. doi: 10.1128/JVI.00207-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30918073 Free PMC article.
-
Novel strategies for inhibiting PD-1 pathway-mediated immune suppression while simultaneously delivering activating signals to tumor-reactive T cells.Cancer Immunol Immunother. 2015 Oct;64(10):1287-93. doi: 10.1007/s00262-015-1677-5. Epub 2015 Mar 20. Cancer Immunol Immunother. 2015. PMID: 25792524 Free PMC article. Review.
-
Targeting T cell costimulation in autoimmune disease.Expert Opin Ther Targets. 2002 Jun;6(3):275-89. doi: 10.1517/14728222.6.3.275. Expert Opin Ther Targets. 2002. PMID: 12223069 Review.
Cited by
-
Landscape and Clinical Significance of Immune Checkpoint in Cutaneous Melanoma.Front Immunol. 2021 Dec 24;12:756282. doi: 10.3389/fimmu.2021.756282. eCollection 2021. Front Immunol. 2021. PMID: 35003069 Free PMC article.
-
Mechanisms of Immunosuppression in Colorectal Cancer.Cancers (Basel). 2020 Dec 20;12(12):3850. doi: 10.3390/cancers12123850. Cancers (Basel). 2020. PMID: 33419310 Free PMC article. Review.
-
Monitoring Circulating Immune Checkpoint Proteins as Predictors of Non-AIDS Morbid Events in People With HIV Initiating Antiretroviral Therapy.Open Forum Infect Dis. 2022 Jan 21;9(3):ofab570. doi: 10.1093/ofid/ofab570. eCollection 2022 Mar. Open Forum Infect Dis. 2022. PMID: 35146038 Free PMC article.
-
Soluble B7-CD28 Family Inhibitory Immune Checkpoint Proteins and Anti-Cancer Immunotherapy.Front Immunol. 2021 Aug 31;12:651634. doi: 10.3389/fimmu.2021.651634. eCollection 2021. Front Immunol. 2021. PMID: 34531847 Free PMC article. Review.
-
Epithelial CD80 promotes immune surveillance of colonic preneoplastic lesions and its expression is increased by oxidative stress through STAT3 in colon cancer cells.J Exp Clin Cancer Res. 2019 May 9;38(1):190. doi: 10.1186/s13046-019-1205-0. J Exp Clin Cancer Res. 2019. PMID: 31072360 Free PMC article.
References
-
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature Med 2002;8:793–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

