Reducing insulin via conditional partial gene ablation in adults reverses diet-induced weight gain
- PMID: 29122848
- PMCID: PMC5892722
- DOI: 10.1096/fj.201700518R
Reducing insulin via conditional partial gene ablation in adults reverses diet-induced weight gain
Abstract
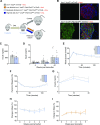
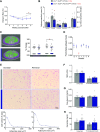
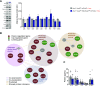
Excess circulating insulin is associated with obesity in humans and in animal models. However, the physiologic causality of hyperinsulinemia in adult obesity has rightfully been questioned because of the absence of clear evidence that weight loss can be induced by acutely reversing diet-induced hyperinsulinemia. Herein, we describe the consequences of inducible, partial insulin gene deletion in a mouse model in which animals have already been made obese by consuming a high-fat diet. A modest reduction in insulin production/secretion was sufficient to cause significant weight loss within 5 wk, with a specific effect on visceral adipose tissue. This result was associated with a reduction in the protein abundance of the lipodystrophy gene polymerase I and transcript release factor ( Ptrf; Cavin) in gonadal adipose tissue. RNAseq analysis showed that reduced insulin and weight loss also associated with a signature of reduced innate immunity. This study demonstrates that changes in circulating insulin that are too fine to adversely affect glucose homeostasis nonetheless exert control over adiposity.-Page, M. M., Skovsø, S., Cen, H., Chiu, A. P., Dionne, D. A., Hutchinson, D. F., Lim, G. E., Szabat, M., Flibotte, S., Sinha, S., Nislow, C., Rodrigues, B., Johnson, J. D. Reducing insulin via conditional partial gene ablation in adults reverses diet-induced weight gain.
Keywords: Ptrf/Cavin; adipose tissue; high-fat diet; innate immunity; obesity.
Conflict of interest statement
The authors thank Farnaz Taghizadeh and Xiaoke (Betty) Hu [both with the University of British Columbia (UBC)] for assistance with islet isolations, perifusions, and animal work; the UBC Sequencing and Bioinformatics Consortium for RNA sequencing; the UBC Centre for High-Throughput Phenogenomics for micro-CT imaging; and John Schipilow and Dr. Nancy Ford (UBC) for technical support and assistance. The Centre is supported by the Canadian Foundation for Innovation, British Columbia Knowledge Development Foundation, and the UBC Faculty of Dentistry. Financial support was provided by an operating grant from the Canadian Institutes of Health Research; Canadian Diabetes Association postdoctoral fellowship awards (to M.M.P. and M.S.); a Canadian Diabetes Association Doctoral Student Research Award (to A.P.C.); a Benzon Foundation Fellowship (to S.S.); and the Juvenile Diabetes Research Foundation and Michael Smith Foundation for Health Research Postdoctoral Fellowship Awards (to G.E.L.). The authors declare no conflicts of interest.
Figures

References
-
- World Health Organization . (2000) Obesity: preventing and managing the global epidemic. WHO Technical Report Series 894 - PubMed
-
- Nolan C. J., Ruderman N. B., Kahn S. E., Pedersen O., Prentki M. (2015) Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes 64, 673–686 https://doi.org/10.2337/db14-0694 - DOI - PMC - PubMed
-
- Kitamura T., Kahn C. R., Accili D. (2003) Insulin receptor knockout mice. Annu. Rev. Physiol. 65, 313–332 https://doi.org/10.1146/annurev.physiol.65.092101.142540 - DOI - PubMed
-
- Gray S. L., Donald C., Jetha A., Covey S. D., Kieffer T. J. (2010) Hyperinsulinemia precedes insulin resistance in mice lacking pancreatic beta-cell leptin signaling. Endocrinology 151, 4178–4186 https://doi.org/10.1210/en.2010-0102 - DOI - PubMed
-
- Templeman N. M., Skovsø S., Page M. M., Lim G. E., Johnson J. D. (2017) A causal role for hyperinsulinemia in obesity. J. Endocrinol. 232, R173–R183 https://doi.org/10.1530/JOE-16-0449 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

