Differential preventive activity of sulindac and atorvastatin in Apc+/Min-FCCCmice with or without colorectal adenomas
- PMID: 29122850
- PMCID: PMC6031273
- DOI: 10.1136/gutjnl-2017-313942
Differential preventive activity of sulindac and atorvastatin in Apc+/Min-FCCCmice with or without colorectal adenomas
Abstract
Objective: The response of subjects to preventive intervention is heterogeneous. The goal of this study was to determine if the efficacy of a chemopreventive agent differs in non-tumour-bearing animals versus those with colorectal tumours. Sulindac and/or atorvastatin was administered to Apc+/Min-FCCC mice with known tumour-bearing status at treatment initiation.
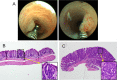
Design: Male mice (6-8 weeks old) underwent colonoscopy and received control chow or chow with sulindac (300 ppm), atorvastatin (100 ppm) or sulindac/atorvastatin. Tissues were collected from mice treated for 14 weeks (histopathology) or 7 days (gene expression). Cell cycle analyses were performed on SW480 colon carcinoma cells treated with sulindac, atorvastatin or both.
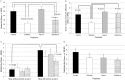
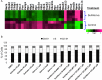
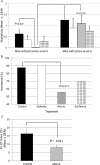
Results: The multiplicity of colorectal adenomas in untreated mice bearing tumours at baseline was 3.6-fold higher than that of mice that were tumour free at baseline (P=0.002). Atorvastatin completely inhibited the formation of microadenomas in mice that were tumour free at baseline (P=0.018) and altered the expression of genes associated with stem/progenitor cells. Treatment of tumour-bearing mice with sulindac/atorvastatin led to a 43% reduction in the multiplicity of colorectal adenomas versus untreated tumour-bearing mice (P=0.049). Sulindac/atorvastatin increased the expression of Hoxb13 and Rprm significantly, suggesting the importance of cell cycle regulation in tumour inhibition. Treatment of SW480 cells with sulindac/atorvastatin led to cell cycle arrest (G0/G1).
Conclusions: The tumour status of animals at treatment initiation dictates response to therapeutic intervention. Atorvastatin eliminated microadenomas in tumour-free mice. The tumour inhibition observed with Sul/Atorva in tumour-bearing mice was greater than that achieved with each agent.
Keywords: cancer prevention; colonoscopy; colorectal adenomas; familial adenomatous polyposis.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
Dysplastic Aberrant Crypt Foci: Biomarkers of Early Colorectal Neoplasia and Response to Preventive Intervention.Cancer Prev Res (Phila). 2020 Mar;13(3):229-240. doi: 10.1158/1940-6207.CAPR-19-0316. Cancer Prev Res (Phila). 2020. PMID: 32132117 Free PMC article. Review.
-
Combined Treatment with a WNT Inhibitor and the NSAID Sulindac Reduces Colon Adenoma Burden in Mice with Truncated APC.Cancer Res Commun. 2022 Feb 2;2(2):66-77. doi: 10.1158/2767-9764.CRC-21-0105. eCollection 2022 Feb. Cancer Res Commun. 2022. PMID: 36860494 Free PMC article.
-
Randomized phase II trial of sulindac, atorvastatin, and prebiotic dietary fiber for colorectal cancer chemoprevention.Cancer Prev Res (Phila). 2011 Feb;4(2):259-69. doi: 10.1158/1940-6207.CAPR-10-0215. Epub 2011 Jan 5. Cancer Prev Res (Phila). 2011. PMID: 21209397 Free PMC article. Clinical Trial.
-
Molecular analysis of sulindac-resistant adenomas in familial adenomatous polyposis.Clin Cancer Res. 2001 Dec;7(12):4000-7. Clin Cancer Res. 2001. PMID: 11751493
-
[Non-steroidal anti-inflammatory agents and prevention of colorectal adenomas and carcinomas].Ugeskr Laeger. 2006 Jan 23;168(4):359-62. Ugeskr Laeger. 2006. PMID: 16436235 Review. Danish. No abstract available.
Cited by
-
Clinically Relevant Anti-Inflammatory Agents for Chemoprevention of Colorectal Cancer: New Perspectives.Int J Mol Sci. 2018 Aug 8;19(8):2332. doi: 10.3390/ijms19082332. Int J Mol Sci. 2018. PMID: 30096840 Free PMC article. Review.
-
The Reprimo Gene Family: A Novel Gene Lineage in Gastric Cancer with Tumor Suppressive Properties.Int J Mol Sci. 2018 Jun 25;19(7):1862. doi: 10.3390/ijms19071862. Int J Mol Sci. 2018. PMID: 29941787 Free PMC article. Review.
-
Dysplastic Aberrant Crypt Foci: Biomarkers of Early Colorectal Neoplasia and Response to Preventive Intervention.Cancer Prev Res (Phila). 2020 Mar;13(3):229-240. doi: 10.1158/1940-6207.CAPR-19-0316. Cancer Prev Res (Phila). 2020. PMID: 32132117 Free PMC article. Review.
-
Combined Treatment with a WNT Inhibitor and the NSAID Sulindac Reduces Colon Adenoma Burden in Mice with Truncated APC.Cancer Res Commun. 2022 Feb 2;2(2):66-77. doi: 10.1158/2767-9764.CRC-21-0105. eCollection 2022 Feb. Cancer Res Commun. 2022. PMID: 36860494 Free PMC article.
-
Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors.Prz Gastroenterol. 2019;14(2):89-103. doi: 10.5114/pg.2018.81072. Epub 2019 Jan 6. Prz Gastroenterol. 2019. PMID: 31616522 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
