Guidelines on the management of abnormal liver blood tests
- PMID: 29122851
- PMCID: PMC5754852
- DOI: 10.1136/gutjnl-2017-314924
Guidelines on the management of abnormal liver blood tests
Abstract
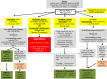
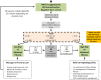
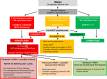
These updated guidelines on the management of abnormal liver blood tests have been commissioned by the Clinical Services and Standards Committee (CSSC) of the British Society of Gastroenterology (BSG) under the auspices of the liver section of the BSG. The original guidelines, which this document supersedes, were written in 2000 and have undergone extensive revision by members of the Guidelines Development Group (GDG). The GDG comprises representatives from patient/carer groups (British Liver Trust, Liver4life, PBC Foundation and PSC Support), elected members of the BSG liver section (including representatives from Scotland and Wales), British Association for the Study of the Liver (BASL), Specialist Advisory Committee in Clinical Biochemistry/Royal College of Pathology and Association for Clinical Biochemistry, British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN), Public Health England (implementation and screening), Royal College of General Practice, British Society of Gastrointestinal and Abdominal Radiologists (BSGAR) and Society of Acute Medicine. The quality of evidence and grading of recommendations was appraised using the AGREE II tool. These guidelines deal specifically with the management of abnormal liver blood tests in children and adults in both primary and secondary care under the following subheadings: (1) What constitutes an abnormal liver blood test? (2) What constitutes a standard liver blood test panel? (3) When should liver blood tests be checked? (4) Does the extent and duration of abnormal liver blood tests determine subsequent investigation? (5) Response to abnormal liver blood tests. They are not designed to deal with the management of the underlying liver disease.
Keywords: alcoholic liver disease; fibrosis; liver; nonalcoholic steatohepatitis.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: PNN: chief investigator for Echosens diagnostic study.
Figures
Comment in
-
Fibrosis-4 (FIB-4) score at the primary care level: an analysis of over 160 000 blood samples.Gut. 2021 Jan;70(1):219-221. doi: 10.1136/gutjnl-2020-320995. Epub 2020 Apr 3. Gut. 2021. PMID: 32245907 No abstract available.
References
-
- Williams R, Aspinall R, Bellis M, et al. . Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014;384:1953–97. 10.1016/S0140-6736(14)61838-9 - DOI - PubMed
-
- Office for National Statistics. Deaths registered in England and Wales (series DR). https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarri....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
