DNPEP is not the only peptidase that produces SPAK fragments in kidney
- PMID: 29122955
- PMCID: PMC5688775
- DOI: 10.14814/phy2.13479
DNPEP is not the only peptidase that produces SPAK fragments in kidney
Abstract
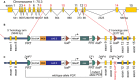
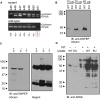
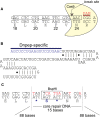
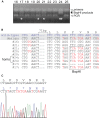
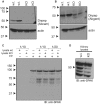
SPAK (STE20/SPS1-related proline/alanine-rich kinase) regulates Na+ and Cl- reabsorption in the distal convoluted tubule, and possibly in the thick ascending limb of Henle. This kinase phosphorylates and activates the apical Na-Cl cotransporter in the DCT. Western blot analysis reveals that SPAK in kidney exists as a full-length protein as well as shorter fragments that might affect NKCC2 function in the TAL. Recently, we showed that kidney lysates exerts proteolytic activity towards SPAK, resulting in the formation of multiple SPAK fragments with possible inhibitory effects on the kinase. The proteolytic activity is mediated by a Zn2+ metalloprotease inhibited by 1,10-phenanthroline, DTT, and EDTA. Size exclusion chromatography demonstrated that the protease was a high-molecular-weight protein. Protein identification by mass-spectrometry analysis after ion exchange and size exclusion chromatography identified multiple proteases as possible candidates and aspartyl aminopeptidase, DNPEP, shared all the properties of the kidney lysate activity. Furthermore, recombinant GST-DNPEP produced similar proteolytic pattern. No mouse knockout model was, however, available to be used as negative control. In this study, we used a DNPEP-mutant mouse generated by EUCOMM as well as a novel CRISPR/cas9 mouse knockout to assess the activity of their kidney lysates towards SPAK. Two mouse models had to be used because different anti-DNPEP antibodies provided conflicting data on whether the EUCOMM mouse resulted in a true knockout. We show that in the absence of DNPEP, the kidney lysates retain their ability to cleave SPAK, indicating that DNPEP might have been misidentified as the protease behind the kidney lysate activity, or that the aspartyl aminopeptidase might not be the only protease cleaving SPAK in kidney.
Keywords: Antibody specificity; CRISPR/cas9; DNPEP knockout; Ste20p‐like kinases; mouse model validation; proteolytic cleavage.
© 2017 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures
Similar articles
-
STE20/SPS1-related proline/alanine-rich kinase (SPAK) is critical for sodium reabsorption in isolated, perfused thick ascending limb.Am J Physiol Renal Physiol. 2015 Mar 1;308(5):F437-43. doi: 10.1152/ajprenal.00493.2013. Epub 2014 Dec 4. Am J Physiol Renal Physiol. 2015. PMID: 25477470 Free PMC article.
-
Short forms of Ste20-related proline/alanine-rich kinase (SPAK) in the kidney are created by aspartyl aminopeptidase (Dnpep)-mediated proteolytic cleavage.J Biol Chem. 2014 Oct 17;289(42):29273-84. doi: 10.1074/jbc.M114.604009. Epub 2014 Aug 27. J Biol Chem. 2014. PMID: 25164821 Free PMC article.
-
SPAK and OSR1 play essential roles in potassium homeostasis through actions on the distal convoluted tubule.J Physiol. 2016 Sep 1;594(17):4945-66. doi: 10.1113/JP272311. Epub 2016 May 29. J Physiol. 2016. PMID: 27068441 Free PMC article.
-
Cotransporters, WNKs and hypertension: an update.Curr Opin Nephrol Hypertens. 2008 Mar;17(2):186-92. doi: 10.1097/MNH.0b013e3282f5244e. Curr Opin Nephrol Hypertens. 2008. PMID: 18277153 Review.
-
SPAK and OSR1, key kinases involved in the regulation of chloride transport.Acta Physiol (Oxf). 2006 May-Jun;187(1-2):103-13. doi: 10.1111/j.1748-1716.2006.01565.x. Acta Physiol (Oxf). 2006. PMID: 16734747 Review.
Cited by
-
C-terminally truncated, kidney-specific variants of the WNK4 kinase lack several sites that regulate its activity.J Biol Chem. 2018 Aug 3;293(31):12209-12221. doi: 10.1074/jbc.RA118.003037. Epub 2018 Jun 19. J Biol Chem. 2018. PMID: 29921588 Free PMC article.
References
-
- Chiga, M. , Rafiqi F. H., Alessi D. R., Sohara E., Ohta A., Rai T., et al. 2011. Phenotypes of pseudohypoaldosteronism type II caused by the WNK4 D561A missense mutation are dependent on the WNK‐OSR1/SPAK kinase cascade. J. Cell Sci. 124:1391–1395. - PubMed
-
- Chowdhury, J. A. , Liu C. H., Zuber A. M., and O'Shaughnessy K. M.. 2013. An inducible transgenic mouse model for familial hypertension with hyperkalaemia (Gordon's syndrome or pseudohypoaldosteronism type II). Clin. Sci. (Lond.) 124:701–708. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

