Organocatalytic enantio- and diastereoselective cycloetherification via dynamic kinetic resolution of chiral cyanohydrins
- PMID: 29123080
- PMCID: PMC5680189
- DOI: 10.1038/s41467-017-01099-x
Organocatalytic enantio- and diastereoselective cycloetherification via dynamic kinetic resolution of chiral cyanohydrins
Abstract
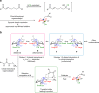
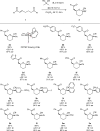
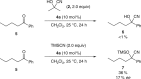
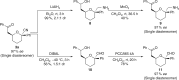
Enantioselective approaches to synthesize six-membered oxacycles with multiple stereogenic centres are in high demand to enable the discovery of new therapeutic agents. Here we present a concise organocatalytic cycloetherification for the highly enantio- and diastereoselective synthesis of tetrahydropyrans involving simultaneous construction of two chiral centres, one of which is fully substituted. This method involves dynamic kinetic resolution of reversibly generated chiral cyanohydrins. A chiral bifunctional organocatalyst selectively recognizes a specific chair-like conformation of the intermediate, in which the small steric effect of the linear cyano group as well as its anomeric effect play important roles in controlling stereoselectivity. The products offer additional utility as synthetic intermediates because the cyano group can be further transformed into a variety of important functional groups. This strategy provides a platform to design efficient approaches to obtain a wide range of optically active tetrahydropyrans, which are otherwise synthetically challenging materials.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Kinetic Resolution of Acylsilane Cyanohydrins via Organocatalytic Cycloetherification.Chem Asian J. 2019 Jan 4;14(1):116-120. doi: 10.1002/asia.201801600. Epub 2018 Nov 27. Chem Asian J. 2019. PMID: 30408346
-
Organocatalytic Enantio- and Diastereoselective Construction of syn-1,3-Diol Motifs via Dynamic Kinetic Resolution of In Situ Generated Chiral Cyanohydrins.Org Lett. 2019 Apr 19;21(8):2688-2692. doi: 10.1021/acs.orglett.9b00677. Epub 2019 Apr 3. Org Lett. 2019. PMID: 30942605
-
Asymmetric Cycloetherification of in Situ Generated Cyanohydrins through the Concomitant Construction of Three Chiral Carbon Centers.Org Lett. 2019 Apr 5;21(7):2156-2160. doi: 10.1021/acs.orglett.9b00462. Epub 2019 Mar 14. Org Lett. 2019. PMID: 30869909
-
Organocatalytic Access to Tetrasubstituted Chiral Carbons Integrating Functional Groups.Chem Rec. 2023 Jul;23(7):e202200200. doi: 10.1002/tcr.202200200. Epub 2022 Sep 26. Chem Rec. 2023. PMID: 36163471 Review.
-
Recent progress on asymmetric organocatalytic construction of chiral cyclohexenone skeletons.Org Biomol Chem. 2014 Apr 28;12(16):2499-513. doi: 10.1039/c3ob42293c. Org Biomol Chem. 2014. PMID: 24599029 Review.
Cited by
-
Site-selective chemical reactions by on-water surface sequential assembly.Nat Commun. 2023 Dec 14;14(1):8313. doi: 10.1038/s41467-023-44129-7. Nat Commun. 2023. PMID: 38097633 Free PMC article.
-
Amine-Catalyzed Decarboxylative Aldol Reaction of β-Ketocarboxylic Acids with Trifluoropyruvates.Molecules. 2019 Jul 30;24(15):2773. doi: 10.3390/molecules24152773. Molecules. 2019. PMID: 31366138 Free PMC article.
-
Harnessing Dpp-Imine as a Powerful Achiral Cocatalyst to Dramatically Increase the Efficiency and Stereoselectivity in a Magnesium-Mediated Oxa-Michael Reaction.JACS Au. 2023 Dec 21;4(1):164-176. doi: 10.1021/jacsau.3c00584. eCollection 2024 Jan 22. JACS Au. 2023. PMID: 38274262 Free PMC article.
-
Organocatalytic kinetic resolution of 1,5-dicarbonyl compounds through a retro-Michael reaction.Beilstein J Org Chem. 2025 Mar 3;21:473-482. doi: 10.3762/bjoc.21.34. eCollection 2025. Beilstein J Org Chem. 2025. PMID: 40079018 Free PMC article.
-
Catalytic Enantioselective Intramolecular Oxa-Michael Reaction to α,β-Unsaturated Esters and Amides.J Am Chem Soc. 2023 Jun 14;145(23):12771-12782. doi: 10.1021/jacs.3c03182. Epub 2023 May 30. J Am Chem Soc. 2023. PMID: 37253087 Free PMC article.
References
-
- Hassel O. Stereochemistry of cyclohexane. Quart. Rev. 1953;7:221–230. doi: 10.1039/qr9530700221. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

