Resistance to cancer immunotherapy mediated by apoptosis of tumor-infiltrating lymphocytes
- PMID: 29123081
- PMCID: PMC5680273
- DOI: 10.1038/s41467-017-00784-1
Resistance to cancer immunotherapy mediated by apoptosis of tumor-infiltrating lymphocytes
Abstract
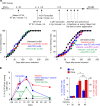
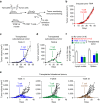
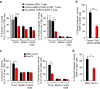
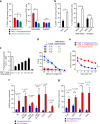
Despite impressive clinical success, cancer immunotherapy based on immune checkpoint blockade remains ineffective in many patients due to tumoral resistance. Here we use the autochthonous TiRP melanoma model, which recapitulates the tumoral resistance signature observed in human melanomas. TiRP tumors resist immunotherapy based on checkpoint blockade, cancer vaccines or adoptive T-cell therapy. TiRP tumors recruit and activate tumor-specific CD8+ T cells, but these cells then undergo apoptosis. This does not occur with isogenic transplanted tumors, which are rejected after adoptive T-cell therapy. Apoptosis of tumor-infiltrating lymphocytes can be prevented by interrupting the Fas/Fas-ligand axis, and is triggered by polymorphonuclear-myeloid-derived suppressor cells, which express high levels of Fas-ligand and are enriched in TiRP tumors. Blocking Fas-ligand increases the anti-tumor efficacy of adoptive T-cell therapy in TiRP tumors, and increases the efficacy of checkpoint blockade in transplanted tumors. Therefore, tumor-infiltrating lymphocytes apoptosis is a relevant mechanism of immunotherapy resistance, which could be blocked by interfering with the Fas/Fas-ligand pathway.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


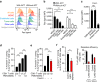

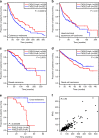
Comment in
-
Back from the dead: TIL apoptosis in cancer immune evasion.Br J Cancer. 2018 Feb 6;118(3):309-311. doi: 10.1038/bjc.2017.483. Epub 2018 Jan 23. Br J Cancer. 2018. PMID: 29360810 Free PMC article. No abstract available.
Similar articles
-
Apoptosis of tumor-infiltrating T lymphocytes: a new immune checkpoint mechanism.Cancer Immunol Immunother. 2019 May;68(5):835-847. doi: 10.1007/s00262-018-2269-y. Epub 2018 Nov 7. Cancer Immunol Immunother. 2019. PMID: 30406374 Free PMC article. Review.
-
Focusing and sustaining the antitumor CTL effector killer response by agonist anti-CD137 mAb.Proc Natl Acad Sci U S A. 2015 Jun 16;112(24):7551-6. doi: 10.1073/pnas.1506357112. Epub 2015 Jun 1. Proc Natl Acad Sci U S A. 2015. PMID: 26034288 Free PMC article.
-
Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation.J Control Release. 2016 Jun 10;231:17-28. doi: 10.1016/j.jconrel.2016.01.044. Epub 2016 Jan 29. J Control Release. 2016. PMID: 26829099
-
Obesity-Associated Myeloid-Derived Suppressor Cells Promote Apoptosis of Tumor-Infiltrating CD8 T Cells and Immunotherapy Resistance in Breast Cancer.Front Immunol. 2020 Oct 6;11:590794. doi: 10.3389/fimmu.2020.590794. eCollection 2020. Front Immunol. 2020. PMID: 33123173 Free PMC article.
-
Tissue-resident memory-like T cells in tumor immunity: Clinical implications.Semin Immunol. 2020 Jun;49:101415. doi: 10.1016/j.smim.2020.101415. Epub 2020 Sep 30. Semin Immunol. 2020. PMID: 33011063 Free PMC article. Review.
Cited by
-
New Perspectives on Myeloid-Derived Suppressor Cells and Their Emerging Role in Haematology.J Clin Med. 2022 Sep 10;11(18):5326. doi: 10.3390/jcm11185326. J Clin Med. 2022. PMID: 36142973 Free PMC article. Review.
-
A Rosetta Stone for Breast Cancer: Prognostic Value and Dynamic Regulation of Neutrophil in Tumor Microenvironment.Front Immunol. 2020 Aug 7;11:1779. doi: 10.3389/fimmu.2020.01779. eCollection 2020. Front Immunol. 2020. PMID: 32849640 Free PMC article. Review.
-
Anti-Apoptotic c-FLIP Reduces the Anti-Tumour Activity of Chimeric Antigen Receptor T Cells.Cancers (Basel). 2022 Oct 4;14(19):4854. doi: 10.3390/cancers14194854. Cancers (Basel). 2022. PMID: 36230780 Free PMC article.
-
Pirin Inhibits FAS-Mediated Apoptosis to Support Colorectal Cancer Survival.Adv Sci (Weinh). 2024 Mar;11(10):e2301476. doi: 10.1002/advs.202301476. Epub 2023 Dec 26. Adv Sci (Weinh). 2024. PMID: 38148593 Free PMC article.
-
Tissue Site and the Cancer Immunity Cycle.Trends Cancer. 2019 Oct;5(10):593-603. doi: 10.1016/j.trecan.2019.07.006. Epub 2019 Aug 13. Trends Cancer. 2019. PMID: 31706507 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

