Supramammillary glutamate neurons are a key node of the arousal system
- PMID: 29123082
- PMCID: PMC5680228
- DOI: 10.1038/s41467-017-01004-6
Supramammillary glutamate neurons are a key node of the arousal system
Abstract
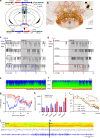
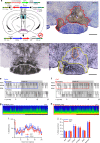
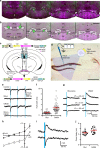
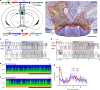
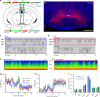
Basic and clinical observations suggest that the caudal hypothalamus comprises a key node of the ascending arousal system, but the cell types underlying this are not fully understood. Here we report that glutamate-releasing neurons of the supramammillary region (SuMvglut2) produce sustained behavioral and EEG arousal when chemogenetically activated. This effect is nearly abolished following selective genetic disruption of glutamate release from SuMvglut2 neurons. Inhibition of SuMvglut2 neurons decreases and fragments wake, also suppressing theta and gamma frequency EEG activity. SuMvglut2 neurons include a subpopulation containing both glutamate and GABA (SuMvgat/vglut2) and another also expressing nitric oxide synthase (SuMNos1/Vglut2). Activation of SuMvgat/vglut2 neurons produces minimal wake and optogenetic stimulation of SuMvgat/vglut2 terminals elicits monosynaptic release of both glutamate and GABA onto dentate granule cells. Activation of SuMNos1/Vglut2 neurons potently drives wakefulness, whereas inhibition reduces REM sleep theta activity. These results identify SuMvglut2 neurons as a key node of the wake-sleep regulatory system.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Economo VonC. Sleep as a problem of localization. J. Nerv. Ment. Dis. 1930;71:249–259. doi: 10.1097/00005053-193003000-00001. - DOI
-
- Ranson SW. Somnolence after hypothalamic lesions in monkeys. Arch. Neurol. Psychiatry. 1939;41:1–23. doi: 10.1001/archneurpsyc.1939.02270130011001. - DOI
-
- Nauta WJH. Hypothalamic regulation of sleep in rats; an experimental study. J. Neurophysiol. 1946;9:285–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

