High-fraction brookite films from amorphous precursors
- PMID: 29123137
- PMCID: PMC5680313
- DOI: 10.1038/s41598-017-15364-y
High-fraction brookite films from amorphous precursors
Abstract
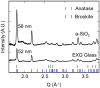
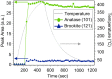
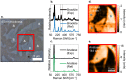
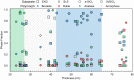
Structure-specific synthesis processes are of key importance to the growth of polymorphic functional compounds such as TiO2, where material properties strongly depend on structure as well as chemistry. The robust growth of the brookite polymorph of TiO2, a promising photocatalyst, has been difficult in both powder and thin-film forms due to the disparity of reported synthesis techniques, their highly specific nature, and lack of mechanistic understanding. In this work, we report the growth of high-fraction (~95%) brookite thin films prepared by annealing amorphous titania precursor films deposited by pulsed laser deposition. We characterize the crystallization process, eliminating the previously suggested roles of substrate templating and Na helper ions in driving brookite formation. Instead, we link phase selection directly to film thickness, offering a novel, generalizable route to brookite growth that does not rely on the presence of extraneous elements or particular lattice-matched substrates. In addition to providing a new synthesis route to brookite thin films, our results take a step towards resolving the problem of phase selection in TiO2 growth, contributing to the further development of this promising functional material.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Liu Q-J, et al. Structural, elastic, electronic and optical properties of various mineral phases of TiO2 from first-principles calculations. Phys. Scripta. 2014;89:075703. doi: 10.1088/0031-8949/89/7/075703. - DOI
-
- Buckeridge J, et al. Polymorph engineering of TiO2: demonstrating how absolute reference potentials are determined by local coordination. Chem. Mater. 2015;27:3644–3851. doi: 10.1021/acs.chemmater.5b00230. - DOI
-
- Hanaor DAH, Sorrell CC. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011;46:855–874. doi: 10.1007/s10853-010-5113-0. - DOI
-
- Di Paola A, Bellardita M, Palmisano L. Brookite, the least known TiO2 photocatalyst. Catalysts. 2013;3:36–73. doi: 10.3390/catal3010036. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

