A nanobody-based tracer targeting DPP6 for non-invasive imaging of human pancreatic endocrine cells
- PMID: 29123178
- PMCID: PMC5680294
- DOI: 10.1038/s41598-017-15417-2
A nanobody-based tracer targeting DPP6 for non-invasive imaging of human pancreatic endocrine cells
Abstract
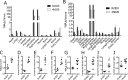
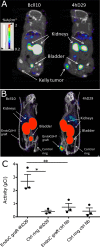
There are presently no reliable ways to quantify endocrine cell mass (ECM) in vivo, which prevents an accurate understanding of the progressive beta cell loss in diabetes or following islet transplantation. To address this unmet need, we coupled RNA sequencing of human pancreatic islets to a systems biology approach to identify new biomarkers of the endocrine pancreas. Dipeptidyl-Peptidase 6 (DPP6) was identified as a target whose mRNA expression is at least 25-fold higher in human pancreatic islets as compared to surrounding tissues and is not changed by proinflammatory cytokines. At the protein level, DPP6 localizes only in beta and alpha cells within the pancreas. We next generated a high-affinity camelid single-domain antibody (nanobody) targeting human DPP6. The nanobody was radiolabelled and in vivo SPECT/CT imaging and biodistribution studies were performed in immunodeficient mice that were either transplanted with DPP6-expressing Kelly neuroblastoma cells or insulin-producing human EndoC-βH1 cells. The human DPP6-expressing cells were clearly visualized in both models. In conclusion, we have identified a novel beta and alpha cell biomarker and developed a tracer for in vivo imaging of human insulin secreting cells. This provides a useful tool to non-invasively follow up intramuscularly implanted insulin secreting cells.
Conflict of interest statement
A.B., I. Ma., S.M., L.B., N.D. and D.L.E. have submitted a patent based on the use of DPP6 and the nanobodies targeting it for pancreatic islet cell imaging. T.L., J.D. and N.D. are cofounders and consultants of the biotech company Camel-IDS. T.L. and N.D. have received funding from Boehringer-Ingelheim, Complix and 121BIO. T.L. and N.D. hold patents on Nanobody imaging and therapy.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

