The liver protection of propylene glycol alginate sodium sulfate preconditioning against ischemia reperfusion injury: focusing MAPK pathway activity
- PMID: 29123239
- PMCID: PMC5680172
- DOI: 10.1038/s41598-017-15521-3
The liver protection of propylene glycol alginate sodium sulfate preconditioning against ischemia reperfusion injury: focusing MAPK pathway activity
Abstract
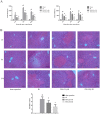
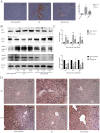
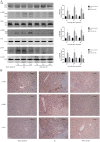
Hepatic ischemia reperfusion (IR) injury contributes to the morbidity and mortality associated with liver surgery. This study investigated the protective function and mechanism of propylene glycol alginate sodium sulfate (PSS), a sulfated polysaccharide, in a mouse hepatic IR injury model. PSS (25 or 50 mg/kg) or saline were injected intraperitoneally to male Balb/c mice 1 h before 45 min of 70% warm hepatic ischemia and 2, 8, and 24 h of reperfusion. Serum and liver tissue samples were collected for evaluation of hepatocellular damage, liver histology, and assay of inflammatory cytokines, apoptosis- and autophagy-related proteins, and proteins in the mitogen-activated protein kinase (MAPKs). Histological injury and release of transaminases, and inflammatory cytokine production were significantly reduced by PSS pretreatment. The expression of apoptosis- and autophagy-related proteins, and the activation of MAPK signal, including jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and P38 were all affected by PSS treatment compared with IR model controls. PSS protected the liver from IR injury by suppressing the MAPK signaling and down-regulating inflammation, apoptosis, and autophagy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Pretreatment with propylene glycol alginate sodium sulfate ameliorated concanavalin A-induced liver injury by regulating the PI3K/Akt pathway in mice.Life Sci. 2017 Sep 15;185:103-113. doi: 10.1016/j.lfs.2017.07.033. Epub 2017 Jul 31. Life Sci. 2017. PMID: 28774703
-
Beraprost sodium preconditioning prevents inflammation, apoptosis, and autophagy during hepatic ischemia-reperfusion injury in mice via the P38 and JNK pathways.Drug Des Devel Ther. 2018 Nov 29;12:4067-4082. doi: 10.2147/DDDT.S182292. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30568428 Free PMC article.
-
Astaxanthin Pretreatment Attenuates Hepatic Ischemia Reperfusion-Induced Apoptosis and Autophagy via the ROS/MAPK Pathway in Mice.Mar Drugs. 2015 May 27;13(6):3368-87. doi: 10.3390/md13063368. Mar Drugs. 2015. PMID: 26023842 Free PMC article.
-
Regulation of autophagy protects against liver injury in liver surgery-induced ischaemia/reperfusion.J Cell Mol Med. 2021 Nov;25(21):9905-9917. doi: 10.1111/jcmm.16943. Epub 2021 Oct 8. J Cell Mol Med. 2021. PMID: 34626066 Free PMC article. Review.
-
Protective Role of mTOR in Liver Ischemia/Reperfusion Injury: Involvement of Inflammation and Autophagy.Oxid Med Cell Longev. 2019 Nov 13;2019:7861290. doi: 10.1155/2019/7861290. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31827701 Free PMC article. Review.
Cited by
-
Novel dihydroartemisinin derivative DHA-37 induces autophagic cell death through upregulation of HMGB1 in A549 cells.Cell Death Dis. 2018 Oct 15;9(11):1048. doi: 10.1038/s41419-018-1006-y. Cell Death Dis. 2018. PMID: 30323180 Free PMC article.
-
TGF-β/Smad and JAK/STAT pathways are involved in the anti-fibrotic effects of propylene glycol alginate sodium sulphate on hepatic fibrosis.J Cell Mol Med. 2020 May;24(9):5224-5237. doi: 10.1111/jcmm.15175. Epub 2020 Mar 31. J Cell Mol Med. 2020. PMID: 32233073 Free PMC article.
-
Mitogen Activated Protein Kinases in Steatotic and Non-Steatotic Livers Submitted to Ischemia-Reperfusion.Int J Mol Sci. 2019 Apr 10;20(7):1785. doi: 10.3390/ijms20071785. Int J Mol Sci. 2019. PMID: 30974915 Free PMC article. Review.
-
Transient Expression of Reck Under Hepatic Ischemia/Reperfusion Conditions Is Associated with Mapk Signaling Pathways.Biomolecules. 2020 May 11;10(5):747. doi: 10.3390/biom10050747. Biomolecules. 2020. PMID: 32403397 Free PMC article.
-
Ubiquitin-Specific Peptidase 10 Protects Against Hepatic Ischaemic/Reperfusion Injury via TAK1 Signalling.Front Immunol. 2020 Sep 29;11:506275. doi: 10.3389/fimmu.2020.506275. eCollection 2020. Front Immunol. 2020. PMID: 33133065 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

