Target-or-Clear Zirconium-89 Labeled Silica Nanoparticles for Enhanced Cancer-Directed Uptake in Melanoma: A Comparison of Radiolabeling Strategies
- PMID: 29123332
- PMCID: PMC5675572
- DOI: 10.1021/acs.chemmater.7b02567
Target-or-Clear Zirconium-89 Labeled Silica Nanoparticles for Enhanced Cancer-Directed Uptake in Melanoma: A Comparison of Radiolabeling Strategies
Abstract
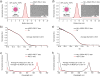
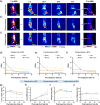
Designing a nanomaterials platform with high target-to-background ratios has long been one of the major challenges in the field of nanomedicine. Here, we introduce a "target-or-clear" multifunctional nanoparticle platform that demonstrates high tumor-targeting efficiency and retention while minimizing off-target effects. Encouraged by the favorable preclinical and clinical pharmacokinetic profiles derived after fine-tuning surface chemical properties of radioiodinated (124I, t1/2 = 100.2 h) ultrasmall cRGDY-conjugated fluorescent silica nanoparticles (C dots), we sought to investigate how the biological properties of these radioconjugates could be influenced by the conjugation of radiometals such as zirconium-89 (89Zr, t1/2 = 78.4 h) using two different strategies: chelator-free and chelator-based radiolabeling. The attachment of 89Zr to newer, surface-aminated, integrin-targeting C' dots using a two-pot synthesis approach led to favorable pharmacokinetics and clearance profiles as well as high tumor uptake and target-to-background ratios in human melanoma models relative to biological controls while maintaining particle sizes below the effective renal glomerular filtration size cutoff <10 nm. Nanoconjugates were also characterized in terms of their radiostability and plasma residence half-lives. Our 89Zr-labeled ultrasmall hybrid organic-inorganic particle is a clinically promising positron emission tomography tracer offering radiobiological properties suitable for enhanced molecularly targeted cancer imaging applications.
Conflict of interest statement
Notes The authors declare no competing financial interest.
Figures





References
-
- Smith BR, Gambhir SS. Nanomaterials for in Vivo Imaging. Chem Rev. 2017;117:901–986. - PubMed
-
- Thakor AS, Gambhir SS. Nanooncology: the Future of Cancer Diagnosis and Therapy. Ca-Cancer J Clin. 2013;63:395–418. - PubMed
-
- Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of Nanoparticle Delivery to Tumours. Nat Rev Mater. 2016;1:16014.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
