A magnetically responsive nanocomposite scaffold combined with Schwann cells promotes sciatic nerve regeneration upon exposure to magnetic field
- PMID: 29123395
- PMCID: PMC5661463
- DOI: 10.2147/IJN.S144715
A magnetically responsive nanocomposite scaffold combined with Schwann cells promotes sciatic nerve regeneration upon exposure to magnetic field
Abstract
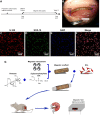
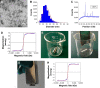
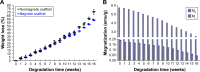
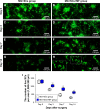
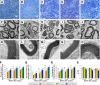
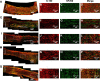
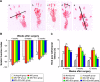
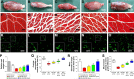
Peripheral nerve repair is still challenging for surgeons. Autologous nerve transplantation is the acknowledged therapy; however, its application is limited by the scarcity of available donor nerves, donor area morbidity, and neuroma formation. Biomaterials for engineering artificial nerves, particularly materials combined with supportive cells, display remarkable promising prospects. Schwann cells (SCs) are the absorbing seeding cells in peripheral nerve engineering repair; however, the attenuated biologic activity restricts their application. In this study, a magnetic nanocomposite scaffold fabricated from magnetic nanoparticles and a biodegradable chitosan-glycerophosphate polymer was made. Its structure was evaluated and characterized. The combined effects of magnetic scaffold (MG) with an applied magnetic field (MF) on the viability of SCs and peripheral nerve injury repair were investigated. The magnetic nanocomposite scaffold showed tunable magnetization and degradation rate. The MGs synergized with the applied MF to enhance the viability of SCs after transplantation. Furthermore, nerve regeneration and functional recovery were promoted by the synergism of SCs-loaded MGs and MF. Based on the current findings, the combined application of MGs and SCs with applied MF is a promising therapy for the engineering of peripheral nerve regeneration.
Keywords: Schwann cell; functional recovery; magnetic field; magnetic nanoparticle; nanocomposite; peripheral nerve repair.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Activation of Schwann cells in vitro by magnetic nanocomposites via applied magnetic field.Int J Nanomedicine. 2014 Dec 17;10:43-61. doi: 10.2147/IJN.S74332. eCollection 2015. Int J Nanomedicine. 2014. PMID: 25565803 Free PMC article.
-
Schwann cell-seeded scaffold with longitudinally oriented micro-channels for reconstruction of sciatic nerve in rats.J Mater Sci Mater Med. 2013 Jul;24(7):1767-80. doi: 10.1007/s10856-013-4917-2. Epub 2013 Mar 20. J Mater Sci Mater Med. 2013. PMID: 23512154
-
Enhanced in vivo survival of Schwann cells by a synthetic oxygen carrier promotes sciatic nerve regeneration and functional recovery.J Tissue Eng Regen Med. 2018 Jan;12(1):e177-e189. doi: 10.1002/term.2284. Epub 2017 Mar 30. J Tissue Eng Regen Med. 2018. PMID: 27592228
-
Transplantation of autologous Schwann cells for the repair of segmental peripheral nerve defects.Neurosurg Focus. 2009 Feb;26(2):E4. doi: 10.3171/FOC.2009.26.2.E4. Neurosurg Focus. 2009. PMID: 19435444 Review.
-
The phenotypic changes of Schwann cells promote the functional repair of nerve injury.Neuropeptides. 2024 Aug;106:102438. doi: 10.1016/j.npep.2024.102438. Epub 2024 May 11. Neuropeptides. 2024. PMID: 38749170 Review.
Cited by
-
A novel bacterial cellulose membrane immobilized with human umbilical cord mesenchymal stem cells-derived exosome prevents epidural fibrosis.Int J Nanomedicine. 2018 Sep 7;13:5257-5273. doi: 10.2147/IJN.S167880. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30237713 Free PMC article.
-
Physical modulation and peripheral nerve regeneration: a literature review.Cell Regen. 2024 Dec 23;13(1):32. doi: 10.1186/s13619-024-00215-9. Cell Regen. 2024. PMID: 39710804 Free PMC article. Review.
-
Magnetic Composite Biomaterials for Neural Regeneration.Front Bioeng Biotechnol. 2019 Jul 25;7:179. doi: 10.3389/fbioe.2019.00179. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31404143 Free PMC article. Review.
-
Microencapsulated Schwann cell transplantation inhibits P2X3 receptor expression in dorsal root ganglia and neuropathic pain.Neural Regen Res. 2018 Nov;13(11):1961-1967. doi: 10.4103/1673-5374.238715. Neural Regen Res. 2018. PMID: 30233070 Free PMC article.
-
Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering.Nanomaterials (Basel). 2021 Sep 8;11(9):2337. doi: 10.3390/nano11092337. Nanomaterials (Basel). 2021. PMID: 34578651 Free PMC article. Review.
References
-
- Sulaiman OA, Gordon T. Effects of short- and long-term schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia. 2000;32(3):234–246. - PubMed
-
- Pabari A, Yang SY, Mosahebi A, et al. Recent advances in artificial nerve conduit design: strategies for the delivery of luminal fillers. J Control Release. 2011;156(1):2–10. - PubMed
-
- Jaquet JB, Luijsterburg AJ, Kalmijn S, et al. Median, ulnar, and combined median-ulnar nerve injuries: functional outcome and return to productivity. J Trauma. 2001;51(4):687–692. - PubMed
-
- Jiang X, Lim SH, Mao HQ, et al. Current applications and future perspectives of artificial nerve conduits. Exp Neurol. 2010;223(1):86–101. - PubMed
-
- Hu X, Huang J, Ye Z, et al. A novel scaffold with longitudinally oriented microchannels promotes peripheral nerve regeneration. Tissue Eng Part A. 2009;15(11):3297–3308. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

