Neutrophil-Derived Interleukin 16 in Premetastatic Lungs Promotes Breast Tumor Cell Seeding
- PMID: 29123422
- PMCID: PMC5661667
- DOI: 10.1177/1179064417738513
Neutrophil-Derived Interleukin 16 in Premetastatic Lungs Promotes Breast Tumor Cell Seeding
Abstract
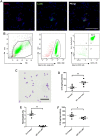
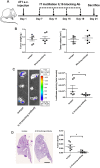
The premetastatic niche in distant organs prior to metastatic cell arrival emerged as an important step in the metastatic cascade. However, molecular mechanisms underlying this process are still poorly understood. In particular, whether neutrophil recruitment at a premetastatic stage promotes or inhibits metastatic cell seeding has to be clarified. We aimed at unraveling how neutrophil infiltration in lung parenchyma induced by the distant primary tumor influences the establishment of lung metastasis. Elevated neutrophil counts and IL-16 levels were found in premetastatic lungs in a syngenic mouse model using 4T1 tumor cells. 4T1 cell-derived soluble factors stimulated IL-16 secretion by neutrophils. The functional contribution of IL-16 is supported by metastasis burden reduction in lungs observed on instillation of an IL-16 neutralizing antibody. Moreover, IL-16 promotes in vitro 4T1 cell adhesiveness, invasiveness, and migration. In conclusion, at a premetastatic stage, neutrophil-derived IL-16 favors tumor cell engraftment in lung parenchyma.
Keywords: IL-16; Tumor microenvironment; breast cancer; lungs; metastasis; neutrophils.
Conflict of interest statement
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.C. is the founder of Aquilon Pharmaceuticals, received speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis, Mundipharma, Chiesi, GSK and received consultancy fees from AstraZeneca, Boehringer Ingelheim, and Novartis for the participation to advisory boards. None of these activities have any connection with oncology or development of drugs in the field of oncology.
Figures




Similar articles
-
Recruited monocytic myeloid-derived suppressor cells promote the arrest of tumor cells in the premetastatic niche through an IL-1β-mediated increase in E-selectin expression.Int J Cancer. 2017 Mar 15;140(6):1370-1383. doi: 10.1002/ijc.30538. Int J Cancer. 2017. PMID: 27885671
-
Unveiling the role of osteosarcoma-derived secretome in premetastatic lung remodelling.J Exp Clin Cancer Res. 2023 Nov 30;42(1):328. doi: 10.1186/s13046-023-02886-9. J Exp Clin Cancer Res. 2023. PMID: 38031171 Free PMC article.
-
Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation.Cancer Cell. 2021 Mar 8;39(3):423-437.e7. doi: 10.1016/j.ccell.2020.12.012. Epub 2021 Jan 14. Cancer Cell. 2021. PMID: 33450198
-
Neutrophils in the premetastatic niche: key functions and therapeutic directions.Mol Cancer. 2024 Sep 14;23(1):200. doi: 10.1186/s12943-024-02107-7. Mol Cancer. 2024. PMID: 39277750 Free PMC article. Review.
-
The metastatic niche formation: focus on extracellular vesicle-mediated dialogue between lung cancer cells and the microenvironment.Front Oncol. 2023 May 3;13:1116783. doi: 10.3389/fonc.2023.1116783. eCollection 2023. Front Oncol. 2023. PMID: 37207158 Free PMC article. Review.
Cited by
-
TRPM2 modulates neutrophil attraction to murine tumor cells by regulating CXCL2 expression.Cancer Immunol Immunother. 2019 Jan;68(1):33-43. doi: 10.1007/s00262-018-2249-2. Epub 2018 Sep 24. Cancer Immunol Immunother. 2019. PMID: 30251149 Free PMC article.
-
Integrated analysis of dysregulated long non-coding RNAs/microRNAs/mRNAs in metastasis of lung adenocarcinoma.J Transl Med. 2018 Dec 27;16(1):372. doi: 10.1186/s12967-018-1732-z. J Transl Med. 2018. PMID: 30587197 Free PMC article.
-
CD62Ldim Neutrophils Specifically Migrate to the Lung and Participate in the Formation of the Pre-Metastatic Niche of Breast Cancer.Front Oncol. 2020 Oct 15;10:540484. doi: 10.3389/fonc.2020.540484. eCollection 2020. Front Oncol. 2020. PMID: 33178575 Free PMC article.
-
Microenvironment-derived ADAM28 prevents cancer dissemination.Oncotarget. 2018 Dec 14;9(98):37185-37199. doi: 10.18632/oncotarget.26449. eCollection 2018 Dec 14. Oncotarget. 2018. PMID: 30647853 Free PMC article.
-
ADAM10 mediates malignant pleural mesothelioma invasiveness.Oncogene. 2019 May;38(18):3521-3534. doi: 10.1038/s41388-018-0669-2. Epub 2019 Jan 16. Oncogene. 2019. PMID: 30651596 Free PMC article.
References
-
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Giordano SH, Buzdar AU, Smith TL, Kau S-W, Yang Y, Hortobagyi GN. Is breast cancer survival improving? Cancer. 2004;100:44–52. - PubMed
-
- Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

