Autoimmunity as a Driving Force of Cognitive Evolution
- PMID: 29123465
- PMCID: PMC5662758
- DOI: 10.3389/fnins.2017.00582
Autoimmunity as a Driving Force of Cognitive Evolution
Abstract
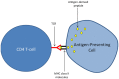
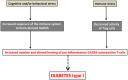
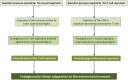
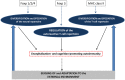
In the last decades, increasingly robust experimental approaches have formally demonstrated that autoimmunity is a physiological process involved in a large range of functions including cognition. On this basis, the recently enunciated "brain superautoantigens" theory proposes that autoimmunity has been a driving force of cognitive evolution. It is notably suggested that the immune and nervous systems have somehow co-evolved and exerted a mutual selection pressure benefiting to both systems. In this two-way process, the evolutionary-determined emergence of neurons expressing specific immunogenic antigens (brain superautoantigens) has exerted a selection pressure on immune genes shaping the T-cell repertoire. Such a selection pressure on immune genes has translated into the emergence of a finely tuned autoimmune T-cell repertoire that promotes cognition. In another hand, the evolutionary-determined emergence of brain-autoreactive T-cells has exerted a selection pressure on neural genes coding for brain superautoantigens. Such a selection pressure has translated into the emergence of a neural repertoire (defined here as the whole of neurons, synapses and non-neuronal cells involved in cognitive functions) expressing brain superautoantigens. Overall, the brain superautoantigens theory suggests that cognitive evolution might have been primarily driven by internal cues rather than external environmental conditions. Importantly, while providing a unique molecular connection between neural and T-cell repertoires under physiological conditions, brain superautoantigens may also constitute an Achilles heel responsible for the particular susceptibility of Homo sapiens to "neuroimmune co-pathologies" i.e., disorders affecting both neural and T-cell repertoires. These may notably include paraneoplastic syndromes, multiple sclerosis as well as autism, schizophrenia and neurodegenerative diseases. In the context of this theoretical frame, a specific emphasis is given here to the potential evolutionary role exerted by two families of genes, namely the MHC class II genes, involved in antigen presentation to T-cells, and the Foxp genes, which play crucial roles in language (Foxp2) and the regulation of autoimmunity (Foxp3).
Keywords: Alzheimer's disease; Parkinson's disease; T-cell repertoire; autism; autoimmunity; cognitive evolution; paraneoplastic syndromes; schizophrenia.
Figures

Similar articles
-
Evolution, immunity and the emergence of brain superautoantigens.F1000Res. 2017 Feb 21;6:171. doi: 10.12688/f1000research.10950.1. eCollection 2017. F1000Res. 2017. PMID: 28529699 Free PMC article.
-
Imprint of thymic selection on autoreactive repertoires.Immunol Rev. 1990 Aug;116:159-70. doi: 10.1111/j.1600-065x.1990.tb00809.x. Immunol Rev. 1990. PMID: 2227993 Review.
-
Thymic commitment of regulatory T cells is a pathway of TCR-dependent selection that isolates repertoires undergoing positive or negative selection.Curr Top Microbiol Immunol. 2005;293:43-71. doi: 10.1007/3-540-27702-1_3. Curr Top Microbiol Immunol. 2005. PMID: 15981475 Review.
-
Gut microbiome and adaptive immunity in schizophrenia.Psychiatriki. 2019 Jul-Sep;30(3):189-192. doi: 10.22365/jpsych.2019.303.189. Psychiatriki. 2019. PMID: 31685450 English, Greek, Modern.
-
Autoimmunity in Parkinson's Disease: The Role of α-Synuclein-Specific T Cells.Front Immunol. 2019 Feb 25;10:303. doi: 10.3389/fimmu.2019.00303. eCollection 2019. Front Immunol. 2019. PMID: 30858851 Free PMC article. Review.
Cited by
-
Philosophical Approach to Neural Autoantibodies in Psychiatric Disease-Multi-Systemic Dynamic Continuum from Protective to Harmful Autoimmunity in Neuronal Systems.Antibodies (Basel). 2022 Dec 23;12(1):1. doi: 10.3390/antib12010001. Antibodies (Basel). 2022. PMID: 36648885 Free PMC article.
-
Interplay Between the Immune and Nervous Cognitive Systems in Homeostasis and in Malaria.Int J Biol Sci. 2023 Jun 28;19(11):3383-3394. doi: 10.7150/ijbs.82556. eCollection 2023. Int J Biol Sci. 2023. PMID: 37496995 Free PMC article. Review.
-
Searching for ancient balanced polymorphisms shared between Neanderthals and Modern Humans.Genet Mol Biol. 2018 Jan-Mar;41(1):67-81. doi: 10.1590/1678-4685-GMB-2017-0308. Genet Mol Biol. 2018. PMID: 29658973 Free PMC article.
-
Elevated Autoantibodies in Subacute Human Spinal Cord Injury Are Naturally Occurring Antibodies.Front Immunol. 2018 Oct 11;9:2365. doi: 10.3389/fimmu.2018.02365. eCollection 2018. Front Immunol. 2018. PMID: 30364218 Free PMC article.
-
The Characterization of Regulatory T-Cell Profiles in Alzheimer's Disease and Multiple Sclerosis.Sci Rep. 2019 Jun 19;9(1):8788. doi: 10.1038/s41598-019-45433-3. Sci Rep. 2019. PMID: 31217537 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

