VGLUT2 Trafficking Is Differentially Regulated by Adaptor Proteins AP-1 and AP-3
- PMID: 29123471
- PMCID: PMC5662623
- DOI: 10.3389/fncel.2017.00324
VGLUT2 Trafficking Is Differentially Regulated by Adaptor Proteins AP-1 and AP-3
Abstract
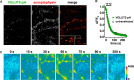
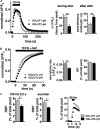
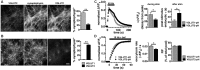
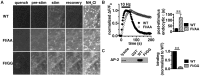
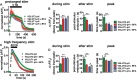
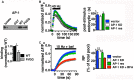
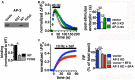
Release of the major excitatory neurotransmitter glutamate by synaptic vesicle exocytosis depends on glutamate loading into synaptic vesicles by vesicular glutamate transporters (VGLUTs). The two principal isoforms, VGLUT1 and 2, exhibit a complementary pattern of expression in adult brain that broadly distinguishes cortical (VGLUT1) and subcortical (VGLUT2) systems, and correlates with distinct physiological properties in synapses expressing these isoforms. Differential trafficking of VGLUT1 and 2 has been suggested to underlie their functional diversity. Increasing evidence suggests individual synaptic vesicle proteins use specific sorting signals to engage specialized biochemical mechanisms to regulate their recycling. We observed that VGLUT2 recycles differently in response to high frequency stimulation than VGLUT1. Here we further explore the trafficking of VGLUT2 using a pHluorin-based reporter, VGLUT2-pH. VGLUT2-pH exhibits slower rates of both exocytosis and endocytosis than VGLUT1-pH. VGLUT2-pH recycling is slower than VGLUT1-pH in both hippocampal neurons, which endogenously express mostly VGLUT1, and thalamic neurons, which endogenously express mostly VGLUT2, indicating that protein identity, not synaptic vesicle membrane or neuronal cell type, controls sorting. We characterize sorting signals in the C-terminal dileucine-like motif, which plays a crucial role in VGLUT2 trafficking. Disruption of this motif abolishes synaptic targeting of VGLUT2 and essentially eliminates endocytosis of the transporter. Mutational and biochemical analysis demonstrates that clathrin adaptor proteins (APs) interact with VGLUT2 at the dileucine-like motif. VGLUT2 interacts with AP-2, a well-studied adaptor protein for clathrin mediated endocytosis. In addition, VGLUT2 also interacts with the alternate adaptors, AP-1 and AP-3. VGLUT2 relies on distinct recycling mechanisms from VGLUT1. Abrogation of these differences by pharmacological and molecular inhibition reveals that these mechanisms are dependent on the adaptor proteins AP-1 and AP-3. Further, shRNA-mediated knockdown reveals differential roles for AP-1 and AP-3 in VGLUT2 recycling.
Keywords: VGLUT; endocytosis; exocytosis; glutamate; synaptic vesicle; vesicular glutamate transporter.
Figures

Similar articles
-
Multiple dileucine-like motifs direct VGLUT1 trafficking.J Neurosci. 2013 Jun 26;33(26):10647-60. doi: 10.1523/JNEUROSCI.5662-12.2013. J Neurosci. 2013. PMID: 23804088 Free PMC article.
-
Inositol hexakisphosphate kinases differentially regulate trafficking of vesicular glutamate transporters 1 and 2.Front Cell Neurosci. 2022 Jul 22;16:926794. doi: 10.3389/fncel.2022.926794. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35936490 Free PMC article.
-
Protein interactions of the vesicular glutamate transporter VGLUT1.PLoS One. 2014 Oct 15;9(10):e109824. doi: 10.1371/journal.pone.0109824. eCollection 2014. PLoS One. 2014. PMID: 25334008 Free PMC article.
-
Trafficking of vesicular neurotransmitter transporters.Traffic. 2008 Sep;9(9):1425-36. doi: 10.1111/j.1600-0854.2008.00771.x. Epub 2008 May 26. Traffic. 2008. PMID: 18507811 Free PMC article. Review.
-
Complementary distribution of vesicular glutamate transporters in the central nervous system.Neurosci Res. 2002 Apr;42(4):243-50. doi: 10.1016/s0168-0102(02)00009-3. Neurosci Res. 2002. PMID: 11985876 Review.
Cited by
-
VGLUT2/Cdk5/p25 Signaling Pathway Contributed to Inflammatory Pain by Complete Freund's Adjuvant.Pain Res Manag. 2020 Feb 29;2020:4807674. doi: 10.1155/2020/4807674. eCollection 2020. Pain Res Manag. 2020. PMID: 32190166 Free PMC article.
-
A Mediation Analysis to Identify Links between Gut Bacteria and Memory in Context of Human Milk Oligosaccharides.Microorganisms. 2021 Apr 15;9(4):846. doi: 10.3390/microorganisms9040846. Microorganisms. 2021. PMID: 33920826 Free PMC article.
-
The mechanism and regulation of vesicular glutamate transport: Coordination with the synaptic vesicle cycle.Biochim Biophys Acta Biomembr. 2020 Dec 1;1862(12):183259. doi: 10.1016/j.bbamem.2020.183259. Epub 2020 Mar 5. Biochim Biophys Acta Biomembr. 2020. PMID: 32147354 Free PMC article. Review.
-
Physiological Perspectives on Molecular Mechanisms and Regulation of Vesicular Glutamate Transport: Lessons From Calyx of Held Synapses.Front Cell Neurosci. 2022 Jan 13;15:811892. doi: 10.3389/fncel.2021.811892. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35095427 Free PMC article. Review.
-
Overcoming presynaptic effects of VAMP2 mutations with 4-aminopyridine treatment.Hum Mutat. 2020 Nov;41(11):1999-2011. doi: 10.1002/humu.24109. Epub 2020 Oct 1. Hum Mutat. 2020. PMID: 32906212 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

