Impact of Vortioxetine on Synaptic Integration in Prefrontal-Subcortical Circuits: Comparisons with Escitalopram
- PMID: 29123483
- PMCID: PMC5662919
- DOI: 10.3389/fphar.2017.00764
Impact of Vortioxetine on Synaptic Integration in Prefrontal-Subcortical Circuits: Comparisons with Escitalopram
Abstract
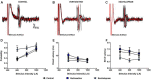
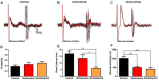
Prefrontal-subcortical circuits support executive functions which often become dysfunctional in psychiatric disorders. Vortioxetine is a multimodal antidepressant that is currently used in the clinic to treat major depressive disorder. Mechanisms of action of vortioxetine include serotonin (5-HT) transporter blockade, 5-HT1A receptor agonism, 5-HT1B receptor partial agonism, and 5-HT1D, 5-HT3, and 5-HT7 receptor antagonism. Vortioxetine facilitates 5-HT transmission in the medial prefrontal cortex (mPFC), however, the impact of this compound on related prefrontal-subcortical circuits is less clear. Thus, the current study examined the impact of systemic vortioxetine administration (0.8 mg/kg, i.v.) on spontaneous spiking and spikes evoked by electrical stimulation of the mPFC in the anterior cingulate cortex (ACC), medial shell of the nucleus accumbens (msNAc), and lateral septal nucleus (LSN) in urethane-anesthetized rats. We also examined whether vortioxetine modulated afferent drive in the msNAc from hippocampal fimbria (HF) inputs. Similar studies were performed using the selective 5-HT reuptake inhibitor [selective serotonin reuptake inhibitors (SSRI)] escitalopram (1.6 mg/kg, i.v.) to enable comparisons between the multimodal actions of vortioxetine and SSRI-mediated effects. No significant differences in spontaneous activity were observed in the ACC, msNAc, and LSN across treatment groups. No significant impact of treatment on mPFC-evoked responses was observed in the ACC. In contrast, vortioxetine decreased mPFC-evoked activity recorded in the msNAc as compared to parallel studies in control and escitalopram treated groups. Thus, vortioxetine may reduce mPFC-msNAc afferent drive via a mechanism that, in addition to an SSRI-like effect, requires 5-HT receptor modulation. Recordings in the LSN revealed a significant increase in mPFC-evoked activity following escitalopram administration as compared to control and vortioxetine treated groups, indicating that complex modulation of 5-HT receptors by vortioxetine may offset SSRI-like effects in this region. Lastly, neurons in the msNAc were more responsive to stimulation of the HF following both vortioxetine and escitalopram administration, indicating that elevation of 5-HT tone and 5-HT receptor modulation may facilitate excitatory hippocampal synaptic drive in this region. The above findings point to complex 5-HT receptor-dependent effects of vortioxetine which may contribute to its unique impact on the function of prefrontal-subcortical circuits and the development of novel strategies for treating mood disorders.
Keywords: cingulate cortex; escitalopram; lateral septum; nucleus accumbens; prefrontal cortex; serotonin; vortioxetine.
Figures


Similar articles
-
Involvement of 5-HT3 receptors in the action of vortioxetine in rat brain: Focus on glutamatergic and GABAergic neurotransmission.Neuropharmacology. 2016 Sep;108:73-81. doi: 10.1016/j.neuropharm.2016.04.023. Epub 2016 Apr 20. Neuropharmacology. 2016. PMID: 27106166
-
Vortioxetine Subchronically Activates Serotonergic Transmission via Desensitization of Serotonin 5-HT1A Receptor with 5-HT3 Receptor Inhibition in Rats.Int J Mol Sci. 2019 Dec 10;20(24):6235. doi: 10.3390/ijms20246235. Int J Mol Sci. 2019. PMID: 31835640 Free PMC article.
-
Lu AA21004, a novel multimodal antidepressant, produces regionally selective increases of multiple neurotransmitters--a rat microdialysis and electrophysiology study.Eur Neuropsychopharmacol. 2013 Feb;23(2):133-45. doi: 10.1016/j.euroneuro.2012.04.006. Epub 2012 May 20. Eur Neuropsychopharmacol. 2013. PMID: 22612991
-
Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data.Pharmacol Ther. 2015 Jan;145:43-57. doi: 10.1016/j.pharmthera.2014.07.001. Epub 2014 Jul 9. Pharmacol Ther. 2015. PMID: 25016186 Review.
-
Can we increase speed and efficacy of antidepressant treatments? Part I: General aspects and monoamine-based strategies.Eur Neuropsychopharmacol. 2018 Apr;28(4):445-456. doi: 10.1016/j.euroneuro.2017.10.032. Epub 2017 Nov 22. Eur Neuropsychopharmacol. 2018. PMID: 29174531 Review.
Cited by
-
Frequency-Specific Changes in the Fractional Amplitude of the Low-Frequency Fluctuations in the Default Mode Network in Medication-Free Patients With Bipolar II Depression: A Longitudinal Functional MRI Study.Front Psychiatry. 2021 Jan 8;11:574819. doi: 10.3389/fpsyt.2020.574819. eCollection 2020. Front Psychiatry. 2021. PMID: 33488415 Free PMC article.
-
Plasticity of synapses and reward circuit function in the genesis and treatment of depression.Neuropsychopharmacology. 2023 Jan;48(1):90-103. doi: 10.1038/s41386-022-01422-1. Epub 2022 Sep 3. Neuropsychopharmacology. 2023. PMID: 36057649 Free PMC article. Review.
-
Predicting escitalopram monotherapy response in depression: The role of anterior cingulate cortex.Hum Brain Mapp. 2020 Apr 1;41(5):1249-1260. doi: 10.1002/hbm.24872. Epub 2019 Nov 22. Hum Brain Mapp. 2020. PMID: 31758634 Free PMC article.
-
Regulation of dopamine neurotransmission from serotonergic neurons by ectopic expression of the dopamine D2 autoreceptor blocks levodopa-induced dyskinesia.Acta Neuropathol Commun. 2019 Jan 15;7(1):8. doi: 10.1186/s40478-018-0653-7. Acta Neuropathol Commun. 2019. PMID: 30646956 Free PMC article.
-
Vortioxetine Differentially Modulates MK-801-Induced Changes in Visual Signal Detection Task Performance and Locomotor Activity.Front Pharmacol. 2018 Sep 13;9:1024. doi: 10.3389/fphar.2018.01024. eCollection 2018. Front Pharmacol. 2018. PMID: 30271344 Free PMC article.
References
-
- Bang-Andersen B., Ruhland T., Jørgensen M., Smith G., Frederiksen K., Jensen K. G., et al. (2011). Discovery of 1-[2-(2, 4-dimethylphenylsulfanyl) phenyl] piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J. Med. Chem. 54 3206–3221. 10.1021/jm101459g - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

