Gonadotropin-Releasing Hormone (GnRH) Receptor Structure and GnRH Binding
- PMID: 29123501
- PMCID: PMC5662886
- DOI: 10.3389/fendo.2017.00274
Gonadotropin-Releasing Hormone (GnRH) Receptor Structure and GnRH Binding
Abstract
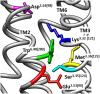
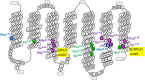
Gonadotropin-releasing hormone (GnRH) regulates reproduction. The human GnRH receptor lacks a cytoplasmic carboxy-terminal tail but has amino acid sequence motifs characteristic of rhodopsin-like, class A, G protein-coupled receptors (GPCRs). This review will consider how recent descriptions of X-ray crystallographic structures of GPCRs in inactive and active conformations may contribute to understanding GnRH receptor structure, mechanism of activation and ligand binding. The structures confirmed that ligands bind to variable extracellular surfaces, whereas the seven membrane-spanning α-helices convey the activation signal to the cytoplasmic receptor surface, which binds and activates heterotrimeric G proteins. Forty non-covalent interactions that bridge topologically equivalent residues in different transmembrane (TM) helices are conserved in class A GPCR structures, regardless of activation state. Conformation-independent interhelical contacts account for a conserved receptor protein structure and their importance in the GnRH receptor structure is supported by decreased expression of receptors with mutations of residues in the network. Many of the GnRH receptor mutations associated with congenital hypogonadotropic hypogonadism, including the Glu2.53(90) Lys mutation, involve amino acids that constitute the conserved network. Half of the ~250 intramolecular interactions in GPCRs differ between inactive and active structures. Conformation-specific interhelical contacts depend on amino acids changing partners during activation. Conserved inactive conformation-specific contacts prevent receptor activation by stabilizing proximity of TM helices 3 and 6 and a closed G protein-binding site. Mutations of GnRH receptor residues involved in these interactions, such as Arg3.50(139) of the DRY/S motif or Tyr7.53(323) of the N/DPxxY motif, increase or decrease receptor expression and efficiency of receptor coupling to G protein signaling, consistent with the native residues stabilizing the inactive GnRH receptor structure. Active conformation-specific interhelical contacts stabilize an open G protein-binding site. Progress in defining the GnRH-binding site has recently slowed, with evidence that Tyr6.58(290) contacts Tyr5 of GnRH, whereas other residues affect recognition of Trp3 and Gly10NH2. The surprisingly consistent observations that GnRH receptor mutations that disrupt GnRH binding have less effect on "conformationally constrained" GnRH peptides may now be explained by crystal structures of agonist-bound peptide receptors. Analysis of GPCR structures provides insight into GnRH receptor function.
Keywords: G protein-coupled receptor; gonadotropin-releasing hormone receptor; ligand binding; receptor activation; receptor structure.
Figures
Similar articles
-
Glu2.53(90) of the GnRH receptor is part of the conserved G protein-coupled receptor structure and does not form a salt-bridge with Lys3.32(121).Mol Cell Endocrinol. 2019 Feb 5;481:53-61. doi: 10.1016/j.mce.2018.11.009. Epub 2018 Nov 23. Mol Cell Endocrinol. 2019. PMID: 30476558
-
Agonist-induced conformational changes in bovine rhodopsin: insight into activation of G-protein-coupled receptors.J Mol Biol. 2008 Oct 3;382(2):539-55. doi: 10.1016/j.jmb.2008.06.084. Epub 2008 Jul 7. J Mol Biol. 2008. PMID: 18638482
-
Histidine(7.36(305)) in the conserved peptide receptor activation domain of the gonadotropin releasing hormone receptor couples peptide binding and receptor activation.Mol Cell Endocrinol. 2015 Feb 15;402:95-106. doi: 10.1016/j.mce.2015.01.008. Epub 2015 Jan 9. Mol Cell Endocrinol. 2015. PMID: 25583361
-
Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function.Pharmacol Ther. 2004 Jul;103(1):21-80. doi: 10.1016/j.pharmthera.2004.05.002. Pharmacol Ther. 2004. PMID: 15251227 Review.
-
7TM Domain Structure of Adhesion GPCRs.Handb Exp Pharmacol. 2016;234:43-66. doi: 10.1007/978-3-319-41523-9_3. Handb Exp Pharmacol. 2016. PMID: 27832483 Review.
Cited by
-
Peptide-Drug Conjugates: Design, Chemistry, and Drug Delivery System as a Novel Cancer Theranostic.ACS Pharmacol Transl Sci. 2024 Jan 24;7(2):309-334. doi: 10.1021/acsptsci.3c00269. eCollection 2024 Feb 9. ACS Pharmacol Transl Sci. 2024. PMID: 38357281 Free PMC article. Review.
-
Understanding COVID-19 Pathogenesis: A Drug-Repurposing Effort to Disrupt Nsp-1 Binding to Export Machinery Receptor Complex.Pathogens. 2021 Dec 17;10(12):1634. doi: 10.3390/pathogens10121634. Pathogens. 2021. PMID: 34959589 Free PMC article.
-
The role of mitogen-activated protein kinase-extracellular receptor kinase pathway in female fertility outcomes: a focus on pituitary gonadotropins regulation.Ther Adv Endocrinol Metab. 2018 Jul;9(7):209-215. doi: 10.1177/2042018818772775. Epub 2018 May 7. Ther Adv Endocrinol Metab. 2018. PMID: 29977499 Free PMC article. Review.
-
Molecular Identification, Characterization, and Expression Analysis of a Gonadotropin-Releasing Hormone Receptor (GnRH-R) in Pacific Abalone, Haliotis discus hannai.Molecules. 2020 Jun 12;25(12):2733. doi: 10.3390/molecules25122733. Molecules. 2020. PMID: 32545589 Free PMC article.
-
Utilizing an Animal Model to Identify Brain Neurodegeneration-Related Biomarkers in Aging.Int J Mol Sci. 2021 Mar 23;22(6):3278. doi: 10.3390/ijms22063278. Int J Mol Sci. 2021. PMID: 33807010 Free PMC article.
References
-
- Struthers RS, Nicholls AJ, Grundy J, Chen T, Jimenez R, Yen SS, et al. Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix. J Clin Endocrinol Metab (2009) 94(2):545–51.10.1210/jc.2008-1695 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

