Lifetimes and spatio-temporal response of protein crystals in intense X-ray microbeams
- PMID: 29123681
- PMCID: PMC5668864
- DOI: 10.1107/S2052252517013495
Lifetimes and spatio-temporal response of protein crystals in intense X-ray microbeams
Abstract
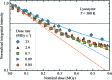
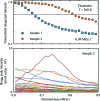
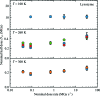
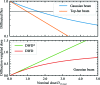
Serial synchrotron-based crystallography using intense microfocused X-ray beams, fast-framing detectors and protein microcrystals held at 300 K promises to expand the range of accessible structural targets and to increase overall structure-pipeline throughputs. To explore the nature and consequences of X-ray radiation damage under microbeam illumination, the time-, dose- and temperature-dependent evolution of crystal diffraction have been measured with maximum dose rates of 50 MGy s-1. At all temperatures and dose rates, the integrated diffraction intensity for a fixed crystal orientation shows non-exponential decays with dose. Non-exponential decays are a consequence of non-uniform illumination and the resulting spatial evolution of diffracted intensity within the illuminated crystal volume. To quantify radiation-damage lifetimes and the damage state of diffracting crystal regions, a revised diffraction-weighted dose (DWD) is defined and it is shown that for Gaussian beams the DWD becomes nearly independent of actual dose at large doses. An apparent delayed onset of radiation damage seen in some intensity-dose curves is in fact a consequence of damage. Intensity fluctuations at high dose rates may arise from the impulsive release of gaseous damage products. Accounting for these effects, data collection at the highest dose rates increases crystal radiation lifetimes near 300 K (but not at 100 K) by a factor of ∼1.5-2 compared with those observed at conventional dose rates. Improved quantification and modeling of the complex spatio-temporal evolution of protein microcrystal diffraction in intense microbeams will enable more efficient data collection, and will be essential in improving the accuracy of structure factors and structural models.
Keywords: X-ray crystallography; intense X-ray microbeams; microcrystallography; protein crystallography; protein structure; radiation damage; serial crystallography; structural biology; structure determination.
Figures
Similar articles
-
Radiation damage and dose limits in serial synchrotron crystallography at cryo- and room temperatures.Proc Natl Acad Sci U S A. 2020 Feb 25;117(8):4142-4151. doi: 10.1073/pnas.1821522117. Epub 2020 Feb 11. Proc Natl Acad Sci U S A. 2020. PMID: 32047034 Free PMC article.
-
Protein microcrystallography using synchrotron radiation.IUCrJ. 2017 Aug 8;4(Pt 5):529-539. doi: 10.1107/S2052252517008193. eCollection 2017 Sep 1. IUCrJ. 2017. PMID: 28989710 Free PMC article. Review.
-
Protein crystal structure from non-oriented, single-axis sparse X-ray data.IUCrJ. 2016 Jan 1;3(Pt 1):43-50. doi: 10.1107/S2052252515018795. eCollection 2016 Jan 1. IUCrJ. 2016. PMID: 26870380 Free PMC article.
-
Room-temperature macromolecular serial crystallography using synchrotron radiation.IUCrJ. 2014 May 30;1(Pt 4):204-12. doi: 10.1107/S2052252514010070. eCollection 2014 Jul 1. IUCrJ. 2014. PMID: 25075341 Free PMC article.
-
Radiation damage to protein specimens from electron beam imaging and diffraction: a mini-review of anti-damage approaches, with special reference to synchrotron X-ray crystallography.J Synchrotron Radiat. 2007 Jan;14(Pt 1):116-27. doi: 10.1107/S0909049506052307. Epub 2006 Dec 15. J Synchrotron Radiat. 2007. PMID: 17211078 Review.
Cited by
-
Determining biomolecular structures near room temperature using X-ray crystallography: concepts, methods and future optimization.Acta Crystallogr D Struct Biol. 2023 Jan 1;79(Pt 1):78-94. doi: 10.1107/S2059798322011652. Epub 2023 Jan 1. Acta Crystallogr D Struct Biol. 2023. PMID: 36601809 Free PMC article.
-
Radiation damage and dose limits in serial synchrotron crystallography at cryo- and room temperatures.Proc Natl Acad Sci U S A. 2020 Feb 25;117(8):4142-4151. doi: 10.1073/pnas.1821522117. Epub 2020 Feb 11. Proc Natl Acad Sci U S A. 2020. PMID: 32047034 Free PMC article.
-
Doses for X-ray and electron diffraction: New features in RADDOSE-3D including intensity decay models.Protein Sci. 2024 Jul;33(7):e5005. doi: 10.1002/pro.5005. Protein Sci. 2024. PMID: 38923423 Free PMC article.
-
High-viscosity injector-based pink-beam serial crystallography of microcrystals at a synchrotron radiation source.IUCrJ. 2019 Apr 5;6(Pt 3):412-425. doi: 10.1107/S205225251900263X. eCollection 2019 May 1. IUCrJ. 2019. PMID: 31098022 Free PMC article.
-
Resolving molecular diffusion and aggregation of antibody proteins with megahertz X-ray free-electron laser pulses.Nat Commun. 2022 Sep 21;13(1):5528. doi: 10.1038/s41467-022-33154-7. Nat Commun. 2022. PMID: 36130930 Free PMC article.
References
-
- Blake, C. C. F. & Phillips, D. C. (1962). Biological Effects of Ionizing Radiation at the Molecular Level, pp. 183–191. Vienna: IAEA.
-
- Botha, S., Nass, K., Barends, T. R. M., Kabsch, W., Latz, B., Dworkowski, F., Foucar, L., Panepucci, E., Wang, M., Shoeman, R. L., Schlichting, I. & Doak, R. B. (2015). Acta Cryst. D71, 387–397. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

