No patient left behind: The promise of immune priming with epigenetic agents
- PMID: 29123948
- PMCID: PMC5665084
- DOI: 10.1080/2162402X.2017.1315486
No patient left behind: The promise of immune priming with epigenetic agents
Abstract
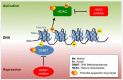
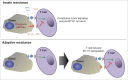
Checkpoint inhibitors, monoclonal antibodies that inhibit PD-1 or CTLA-4, have revolutionized the treatment of multiple cancers. Despite the enthusiasm for the clinical successes of checkpoint inhibitors, and immunotherapy, in general, only a minority of patients with specific tumor types actually benefit from treatment. Emerging evidence implicates epigenetic alterations as a mechanism of clinical resistance to immunotherapy. This review presents evidence for that association, summarizes the epi-based mechanisms by which tumors evade immunogenic cell death, discusses epigenetic modulation as a component of an integrated strategy to boost anticancer T cell effector function in relation to a tumor immunosuppression cycle and, finally, makes the case that the success of this no-patient-left-behind strategy critically depends on the toxicity profile of the epigenetic agent(s).
Keywords: Immunotherapy; checkpoint inhibitors; epigenetic modulation; immunogenic cell death; immunosuppression; resistance.
Figures




Similar articles
-
Immune Checkpoint Blockade in Breast Cancer Therapy.Adv Exp Med Biol. 2017;1026:383-402. doi: 10.1007/978-981-10-6020-5_18. Adv Exp Med Biol. 2017. PMID: 29282694 Review.
-
Recent advances in the clinical development of immune checkpoint blockade therapy.Cell Oncol (Dordr). 2019 Oct;42(5):609-626. doi: 10.1007/s13402-019-00456-w. Epub 2019 Jun 14. Cell Oncol (Dordr). 2019. PMID: 31201647 Review.
-
Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies.Int Immunol. 2015 Jan;27(1):39-46. doi: 10.1093/intimm/dxu095. Epub 2014 Oct 16. Int Immunol. 2015. PMID: 25323844 Review.
-
Innovative Therapy, Monoclonal Antibodies and Beyond.Cytokine Growth Factor Rev. 2017 Dec;38:1-9. doi: 10.1016/j.cytogfr.2017.10.002. Epub 2017 Oct 5. Cytokine Growth Factor Rev. 2017. PMID: 29029813
-
From a Patient Advocate's Perspective: Does Cancer Immunotherapy Represent a Paradigm Shift?Curr Oncol Rep. 2018 Feb 7;20(1):8. doi: 10.1007/s11912-018-0662-5. Curr Oncol Rep. 2018. PMID: 29411148 Review.
Cited by
-
The epigenetic immunomodulator, HBI-8000, enhances the response and reverses resistance to checkpoint inhibitors.BMC Cancer. 2021 Aug 30;21(1):969. doi: 10.1186/s12885-021-08702-x. BMC Cancer. 2021. PMID: 34461854 Free PMC article.
-
Trial watch: Peptide-based vaccines in anticancer therapy.Oncoimmunology. 2018 Sep 6;7(12):e1511506. doi: 10.1080/2162402X.2018.1511506. eCollection 2018. Oncoimmunology. 2018. PMID: 30524907 Free PMC article. Review.
-
CCDC6 and USP7 expression levels suggest novel treatment options in high-grade urothelial bladder cancer.J Exp Clin Cancer Res. 2019 Feb 20;38(1):90. doi: 10.1186/s13046-019-1087-1. J Exp Clin Cancer Res. 2019. PMID: 30786932 Free PMC article.
-
Current Strategies to Enhance Anti-Tumour Immunity.Biomedicines. 2018 Mar 23;6(2):37. doi: 10.3390/biomedicines6020037. Biomedicines. 2018. PMID: 29570634 Free PMC article. Review.
-
The transition from primary colorectal cancer to isolated peritoneal malignancy is associated with an increased tumour mutational burden.Sci Rep. 2020 Nov 3;10(1):18900. doi: 10.1038/s41598-020-75844-6. Sci Rep. 2020. PMID: 33144643 Free PMC article.
References
-
- Coley WB., II Contribution to the knowledge of sarcoma. Ann Surg 1891; 14:199-220; PMID:17859590; https://doi.org/10.1097/00000658-189112000-00015 - DOI - PMC - PubMed
-
- Parish CR. Cancer immunotherapy: The past, the present and the future. Immunol Cell Biol 2003; 81:106-13; PMID:12631233; https://doi.org/10.1046/j.0818-9641.2003.01151.x - DOI - PubMed
-
- Wiemann B, Starnes CO. Coley's toxins, tumor necrosis factor and cancer research: A historical perspective. Pharmacol Ther 1994; 64:529-64; PMID:7724661; https://doi.org/10.1016/0163-7258(94)90023-X - DOI - PubMed
-
- Karbach J, Neumann A, Brand K, Wahle C, Siegel E, Maeurer M, Ritter E, Tsuji T, Gnjatic S, Old LJ, et al.. Phase I clinical trial of mixed bacterial vaccine (Coley's toxins) in patients with NY-ESO-1 expressing cancers: Immunological effects and clinical activity. Clin Cancer Res 2012; 18:5449-59; PMID:22847809; https://doi.org/10.1158/1078-0432.CCR-12-1116 - DOI - PubMed
-
- Firor AE, Jares A, Ma Y. From humble beginnings to success in the clinic: Chimeric antigen receptor-modified T-cells and implications for immunotherapy. Exp Biol Med (Maywood) 2015; 240:1087-98; PMID:25956686; https://doi.org/10.1177/1535370215584936 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
