Characterization of a switchable chimeric antigen receptor platform in a pre-clinical solid tumor model
- PMID: 29123951
- PMCID: PMC5665068
- DOI: 10.1080/2162402X.2017.1342909
Characterization of a switchable chimeric antigen receptor platform in a pre-clinical solid tumor model
Abstract
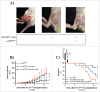
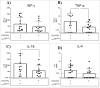
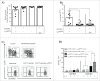
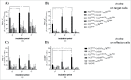
The universal modular chimeric antigen receptor (UniCAR) platform redirects CAR-T cells using a separated, soluble targeting module with a short half-life. This segregation allows precise controllability and flexibility. Herein we show that the UniCAR platform can be used to efficiently target solid cancers in vitro and in vivo using a pre-clinical prostate cancer model which overexpresses prostate stem cell antigen (PSCA). Short-term administration of the targeting module to tumor bearing immunocompromised mice engrafted with human UniCAR-T cells significantly delayed tumor growth and prolonged survival of recipient mice both in a low and high tumor burden model. In addition, we analyzed phenotypic and functional changes of cancer cells and UniCAR-T cells in association with the administration of the targeting module to reveal potential immunoevasive mechanisms. Most notably, UniCAR-T cell activation induced upregulation of immune-inhibitory molecules such as programmed death ligands. In conclusion, this work illustrates that the UniCAR platform mediates potent anti-tumor activity in a relevant in vitro and in vivo solid tumor model.
Keywords: Chimeric antigen receptors; immune checkpoints; immunoevasion; prostate stem cell antigen; solid tumors; targeting module.
Figures


Similar articles
-
Evaluation of switch-mediated costimulation in trans on universal CAR-T cells (UniCAR) targeting CD123-positive AML.Oncoimmunology. 2021 Jul 8;10(1):1945804. doi: 10.1080/2162402X.2021.1945804. eCollection 2021. Oncoimmunology. 2021. PMID: 34290907 Free PMC article.
-
Extended half-life target module for sustainable UniCAR T-cell treatment of STn-expressing cancers.J Exp Clin Cancer Res. 2020 May 5;39(1):77. doi: 10.1186/s13046-020-01572-4. J Exp Clin Cancer Res. 2020. PMID: 32370811 Free PMC article.
-
Rapidly Switchable Universal CAR-T Cells for Treatment of CD123-Positive Leukemia.Mol Ther Oncolytics. 2020 Apr 29;17:408-420. doi: 10.1016/j.omto.2020.04.009. eCollection 2020 Jun 26. Mol Ther Oncolytics. 2020. PMID: 32462078 Free PMC article.
-
Engineering switchable and programmable universal CARs for CAR T therapy.J Hematol Oncol. 2019 Jul 4;12(1):69. doi: 10.1186/s13045-019-0763-0. J Hematol Oncol. 2019. PMID: 31272471 Free PMC article. Review.
-
The UniCAR system: A modular CAR T cell approach to improve the safety of CAR T cells.Immunol Lett. 2019 Jul;211:13-22. doi: 10.1016/j.imlet.2019.05.003. Epub 2019 May 12. Immunol Lett. 2019. PMID: 31091431 Review.
Cited by
-
Evaluation of switch-mediated costimulation in trans on universal CAR-T cells (UniCAR) targeting CD123-positive AML.Oncoimmunology. 2021 Jul 8;10(1):1945804. doi: 10.1080/2162402X.2021.1945804. eCollection 2021. Oncoimmunology. 2021. PMID: 34290907 Free PMC article.
-
The antigen-binding moiety in the driver's seat of CARs.Med Res Rev. 2022 Jan;42(1):306-342. doi: 10.1002/med.21818. Epub 2021 May 24. Med Res Rev. 2022. PMID: 34028069 Free PMC article. Review.
-
Influencing factors and solution strategies of chimeric antigen receptor T-cell therapy (CAR-T) cell immunotherapy.Oncol Res. 2024 Aug 23;32(9):1479-1516. doi: 10.32604/or.2024.048564. eCollection 2024. Oncol Res. 2024. PMID: 39220130 Free PMC article. Review.
-
From mono- to bivalent: improving theranostic properties of target modules for redirection of UniCAR T cells against EGFR-expressing tumor cells in vitro and in vivo.Oncotarget. 2018 May 22;9(39):25597-25616. doi: 10.18632/oncotarget.25390. eCollection 2018 May 22. Oncotarget. 2018. PMID: 29876011 Free PMC article.
-
Modular (universal) CAR-T platforms in vivo: a comprehensive systematic review.Front Immunol. 2024 Dec 6;15:1409665. doi: 10.3389/fimmu.2024.1409665. eCollection 2024. Front Immunol. 2024. PMID: 39712013 Free PMC article.
References
-
- Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA 1989; 86:10024-28; PMID:2513569; https://doi.org/10.1073/pnas.86.24.10024 - DOI - PMC - PubMed
-
- Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol 2002; 20:70-5; PMID:11753365; https://doi.org/10.1038/nbt0102-70 - DOI - PubMed
-
- Sadelain M, Brentjens R, Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol 2009; 21:215-23; PMID:19327974; https://doi.org/10.1016/j.coi.2009.02.009 - DOI - PMC - PubMed
-
- Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O, et al.. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011; 118:4817-28; PMID:21849486; https://doi.org/10.1182/blood-2011-04-348540 - DOI - PMC - PubMed
-
- Brentjens RJ, Davila ML, Rivèire I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, et al.. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5:177ra38; PMID:23515080; https://doi.org/10.1126/scitranslmed.3005930 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
