Mevalonate metabolism governs cancer immune surveillance
- PMID: 29123952
- PMCID: PMC5665080
- DOI: 10.1080/2162402X.2017.1342917
Mevalonate metabolism governs cancer immune surveillance
Abstract
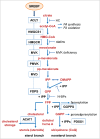
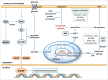
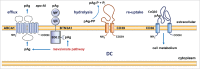
The metabolic reprogramming that drives immunity engages the mevalonate pathway for cholesterol biosynthesis and protein prenylation. The importance of tight regulation of this metabolic route is reflected by the fact that too low activity impairs cellular function and survival, whereas hyperactivity can lead to malignant transformation. Here, we first address how mevalonate metabolism drives immunity and then highlight ways of the immune system to respond to both, limited and uncontrolled flux through the mevalonate pathway. Immune responses elicited by mevalonate pathway dysregulation may be harnessed to increase the clinical efficacy of current cancer therapy regimens.
Keywords: ATP citrate lyase; HMG-CoA reductase; T lymphocytes; cancer; cholesterol; dendritic cells; flux; immunometabolism; macrophages; mevalonate; protein prenylation.
Figures

References
-
- Motulsky AG. The 1985 Nobel Prize in physiology or medicine. Science 1986; 231:126-9; PMID:3510453; https://doi.org/10.1126/science.3510453 - DOI - PubMed
-
- O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol 2016; 16:553-65; PMID:27396447; https://doi.org/10.1038/nri.2016.70 - DOI - PMC - PubMed
-
- Murray PJ, Rathmell J, Pearce E. SnapShot: Immunometabolism. Cell Metab 2015; 22:190-e1; PMID:26154058; https://doi.org/10.1016/j.cmet.2015.06.014 - DOI - PubMed
-
- Buck MD, O'Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med 2015; 212:1345-60; PMID:26261266; https://doi.org/10.1084/jem.20151159 - DOI - PMC - PubMed
-
- MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol 2013; 31:259-83; PMID:23298210; https://doi.org/10.1146/annurev-immunol-032712-095956 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
