Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis
- PMID: 29123955
- PMCID: PMC5665065
- DOI: 10.1080/2162402X.2017.1344805
Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis
Abstract
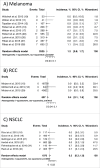
Background: With the rising use of immune checkpoint inhibitors (ICI) across varying tumors types, immune-related colitis is an increasingly encountered, serious adverse event requiring appropriate management. The incidence across ICI treatment regimens and tumor types is unclear. Objective: To characterize the incidence of immune-related colitis among various ICI regimens and tumor types. Methods: Thirty-four original studies of prospective ICI trials were identified based on a PubMed search completed on November 1st, 2016. Seventeen studies compared incidences across tumor types. The incidences of all-grade, grade 3-4 (severe) colitis, and grade 3-4 (severe) diarrhea were collected. Results: Thirty-four studies containing 8863 patients were included in the meta-analysis. The overall incidence during ipilimumab monotherapy was 9.1% for all-grade colitis, 6.8% for severe colitis, and 7.9% for severe diarrhea. The incidence was lowest during PD-1/PD-L1 inhibitor monotherapy with 1.3% for all-grade colitis, 0.9% for severe colitis and 1.2% for severe diarrhea, while combination ipilimumab and nivolumab resulted in the highest incidences of all-grade colitis (13.6%), severe colitis (9.4%) and severe diarrhea (9.2%) among ICIs. Among melanoma, NSCLC, RCC patients, incidences of colitis and diarrhea with PD-1/PD-L1 inhibitor monotherapy did not significantly differ. Severe colitis incidence was similar with ipilimumab monotherapy at 3 mg/kg and 10 mg/kg (7.1% vs 5.1%, respectively), but significantly higher for severe diarrhea with 10mg/kg (11.5% vs 5.2%). Conclusions: The incidence of immune-related colitis and severe diarrhea was higher with ipilimumab-containing regimens compared with PD-1/PD-L1 inhibitors. There was no significant difference in immune-related colitis between different tumor types with PD-1/L1 inhibitors.
Keywords: Immunotherapy; immune-related colitis; melanoma; meta-analysis; non-small cell lung cancer; renal cell carcinoma.
Figures


References
-
- Weber JS, O'Day S, Urba W, Powderly J, Nichol G, Yellin M, Snively J, Hersh E. Phase i/ii study of ipilimumab for patients with metastatic melanoma. J Clin Oncol 2008; 26(36):5950-56; PMID:19018089; https://doi.org/ 10.1200/JCO.2008.16.1927 - DOI - PubMed
-
- Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, Ridolfi R, Assi H, Maraveyas A, Berman D, et al.. A randomized, double-blind, placebo-controlled, phase ii study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage iii or iv melanoma. Clin Cancer Res 2009; 15(17):5591-98; PMID:19671877; https://doi.org/ 10.1158/1078-0432.CCR-09-1024 - DOI - PubMed
-
- Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T Jr. et al.. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010; 11(2):155-64; PMID:20004617; https://doi.org/ 10.1016/S1470-2045(09)70334-1 - DOI - PubMed
-
- O'Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, Queirolo P, Lundgren L, Mikhailov S, Roman L, et al.. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: A multicenter single-arm phase ii study. Ann Oncol 2010; 21(8):1712-17; https://doi.org/ 10.1093/annonc/mdq013 - DOI - PubMed
-
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al.. Phase i study of single-agent anti-programmed death-1 (mdx-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28(19):3167-75; PMID:20516446; https://doi.org/ 10.1200/JCO.2009.26.7609 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
