Mutations in JAK2 and Calreticulin genes are associated with specific alterations of the immune system in myelofibrosis
- PMID: 29123956
- PMCID: PMC5665081
- DOI: 10.1080/2162402X.2017.1345402
Mutations in JAK2 and Calreticulin genes are associated with specific alterations of the immune system in myelofibrosis
Abstract
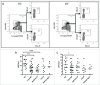
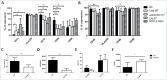
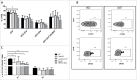
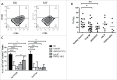
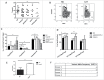
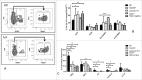
Myelofibrosis (MF) is a clonal neoplasia associated with chronic inflammation due to aberrant cytokine production. Mutations in Janus Kinase-2 (JAK2), calreticulin (CALR) and myeloproliferative leukemia protein (MPL) genes have been recently associated to MF and they all activate the JAK/STAT signaling pathway. Since this pathway is essential in shaping the immune response, we investigated the role of circulating immune subsets and cytokines in 38 patients (20 carrying JAK2(V617F),13 exon-9 CALR mutation and 5 triple negative). In comparison to healthy donors, patients presented a reduced amount of circulating dendritic cells (DCs) associated with a defective ability of monocytes in differentiating into DCs. In addition, we found a reduction in circulating T-helper (Th)1 and Th17 and hypo-functional innate lymphoid cells (ILC). Results analyzed according to the mutational status showed that patients carrying JAK2(V617F) mutation had a reduction in Th17, myeloid-DCs and effector Tregs as well as increased ILC1 and cytokine producing Tregs. The CALR mutated patients revealed high ILC3 levels, reduced Th1 and their monocytes had a reduced capacity to mature in vitro into fully committed DCs. Their Tregs were also less effective in inhibiting the proliferation of autologous effector T-cells due to an increased proliferative status induced by CALR mutation. Triple negative patients presented a reduced amount of total circulating CD3, effectors Tregs and Th1 with increased ILC1. Overall, we have demonstrated that in MF different mutations lead to phenotypic and functional alterations in different immune subsets that may have a potential role in disease progression and susceptibility to infections.
Keywords: Calreticulin; Immune dysregulation; JAK2(V617F); Myelofibrosis; chronic inflammation.
Figures
Similar articles
-
Changing concepts of diagnostic criteria of myeloproliferative disorders and the molecular etiology and classification of myeloproliferative neoplasms: from Dameshek 1950 to Vainchenker 2005 and beyond.Acta Haematol. 2015;133(1):36-51. doi: 10.1159/000358580. Epub 2014 Aug 7. Acta Haematol. 2015. PMID: 25116092 Review.
-
Somatic mutations of calreticulin in myeloproliferative neoplasms.N Engl J Med. 2013 Dec 19;369(25):2379-90. doi: 10.1056/NEJMoa1311347. Epub 2013 Dec 10. N Engl J Med. 2013. PMID: 24325356
-
[Analysis of CALR, JAK2 and MPL gene mutations in BCR-ABL negative myeloproliferative neoplasms].Zhonghua Yi Xue Za Zhi. 2015 May 12;95(18):1369-73. Zhonghua Yi Xue Za Zhi. 2015. PMID: 26178351 Chinese.
-
An accurate, simple prognostic model consisting of age, JAK2, CALR, and MPL mutation status for patients with primary myelofibrosis.Haematologica. 2017 Jan;102(1):79-84. doi: 10.3324/haematol.2016.149765. Epub 2016 Sep 29. Haematologica. 2017. PMID: 27686378 Free PMC article.
-
Aspects biologiques de la voie JAK/STAT dans les néoplasmes myéloprolifératifs classiques négatifs pour BCR-ABL.Bull Cancer. 2016 Jun;103(6 Suppl 1):S16-28. doi: 10.1016/S0007-4551(16)30142-4. Bull Cancer. 2016. PMID: 27494969 Review. French.
Cited by
-
The Dual Role of Innate Lymphoid and Natural Killer Cells in Cancer. from Phenotype to Single-Cell Transcriptomics, Functions and Clinical Uses.Cancers (Basel). 2021 Oct 9;13(20):5042. doi: 10.3390/cancers13205042. Cancers (Basel). 2021. PMID: 34680190 Free PMC article. Review.
-
Distinct profile of CD34+ cells and plasma-derived extracellular vesicles from triple-negative patients with Myelofibrosis reveals potential markers of aggressive disease.J Exp Clin Cancer Res. 2021 Feb 1;40(1):49. doi: 10.1186/s13046-020-01776-8. J Exp Clin Cancer Res. 2021. PMID: 33522952 Free PMC article.
-
Inflammatory Microenvironment and Specific T Cells in Myeloproliferative Neoplasms: Immunopathogenesis and Novel Immunotherapies.Int J Mol Sci. 2021 Feb 14;22(4):1906. doi: 10.3390/ijms22041906. Int J Mol Sci. 2021. PMID: 33672997 Free PMC article. Review.
-
Genetics and Pathogenetic Role of Inflammasomes in Philadelphia Negative Chronic Myeloproliferative Neoplasms: A Narrative Review.Int J Mol Sci. 2021 Jan 8;22(2):561. doi: 10.3390/ijms22020561. Int J Mol Sci. 2021. PMID: 33429941 Free PMC article. Review.
-
Assessment of effects of repeated oral doses of fedratinib on inhibition of cytochrome P450 activities in patients with solid tumors using a cocktail approach.Cancer Chemother Pharmacol. 2020 Jul;86(1):87-95. doi: 10.1007/s00280-020-04102-3. Epub 2020 Jun 14. Cancer Chemother Pharmacol. 2020. PMID: 32537715 Clinical Trial.
References
-
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, et al.. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114:937-51; https://doi.org/10.1182/blood-2009-03-209262 - DOI - PubMed
-
- James C, Ugo V, Casadevall N, Constantinescu SN, Vainchenker W. A JAK2 mutation in myeloproliferative disorders: pathogenesis and therapeutic and scientific prospects. Trends Mol Med 2005; 11:546-54; https://doi.org/10.1016/j.molmed.2005.10.003 - DOI - PubMed
-
- Lavi N. Calreticulin mutations in myeloproliferative neoplasms. Rambam Maimonides Med J 2014; 5:e0035; PMID:25386351; https://doi.org/10.5041/RMMJ.10169 - DOI - PMC - PubMed
-
- Rampal R, Al-Shahrour F, Abdel-Wahab O, Patel JP, Brunel JP, Mermel CH, Bass AJ, Pretz J, Ahn J, Hricik T, et al.. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood 2014; 123:e123-33; PMID:24740812; https://doi.org/10.1182/blood-2014-02-554634 - DOI - PMC - PubMed
-
- Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, Guglielmelli P, Pungolino E, Caramella M, Maffioli M, et al.. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 2010; 115:1703-8; PMID:20008785; https://doi.org/10.1182/blood-2009-09-245837 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
