Immunological efficacy of glypican-3 peptide vaccine in patients with advanced hepatocellular carcinoma
- PMID: 29123959
- PMCID: PMC5665076
- DOI: 10.1080/2162402X.2017.1346764
Immunological efficacy of glypican-3 peptide vaccine in patients with advanced hepatocellular carcinoma
Abstract
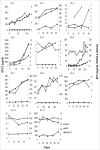
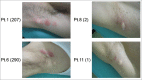
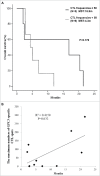
We have previously conducted a phase I trial to test the efficacy of a glypican-3 (GPC3) peptide vaccine in patients with advanced hepatocellular carcinoma (HCC); however, its immunological mechanism of action remains unclear. Here, we report a pilot study conducted to evaluate the immunological mechanisms of action of this GPC3 peptide vaccine (UMIN-CTR number 000005093). Eleven patients with advanced HCC were vaccinated with the GPC3 peptide in this trial. The primary end point was GPC3 peptide-specific immune response induced by the GPC3 peptide vaccination. The secondary endpoints were clinical and biologic outcomes. We demonstrated that the present vaccine induced GPC3 peptide-specific cytotoxic T lymphocytes (CTLs), which were found to infiltrate into the tumor. Moreover, we established GPC3 peptide-specific CTL clones from a biopsy specimen: these cells exhibited GPC3 peptide-specific cytokine secretion and cell cytotoxicity. The plasma GPC3 level tended to decrease temporarily at least once during the follow-up period. The GPC3-specific CTL frequency after vaccination was correlated with overall survival. The degree of skin reactions at the injection site correlated with the GPC3 peptide-specific CTLs. Furthermore, we sequenced the T cell receptors (TCRs) of tumor-infiltrating lymphocyte (TIL) clones, and confirmed the existence of this TCR repertoire in both tumor tissue and PBMCs. In response to these data, we are developing TCR-engineered T cell therapy using TCR sequences obtained from GPC3 peptide-specific CTL clones for improved efficacy in patients with advanced HCC.
Keywords: CTL; Glypican-3 (GPC3); HCC; Peptide vaccine; immunological efficacy.
Figures

Similar articles
-
HLA-A2-restricted glypican-3 peptide-specific CTL clones induced by peptide vaccine show high avidity and antigen-specific killing activity against tumor cells.Cancer Sci. 2011 May;102(5):918-25. doi: 10.1111/j.1349-7006.2011.01896.x. Epub 2011 Mar 4. Cancer Sci. 2011. PMID: 21281401 Free PMC article. Clinical Trial.
-
Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival.Clin Cancer Res. 2012 Jul 1;18(13):3686-96. doi: 10.1158/1078-0432.CCR-11-3044. Epub 2012 May 10. Clin Cancer Res. 2012. PMID: 22577059 Clinical Trial.
-
Analysis of cytotoxic T lymphocytes from a patient with hepatocellular carcinoma who showed a clinical response to vaccination with a glypican‑3‑derived peptide.Int J Oncol. 2013 Oct;43(4):1019-26. doi: 10.3892/ijo.2013.2044. Epub 2013 Jul 31. Int J Oncol. 2013. PMID: 23903757 Free PMC article.
-
[The cancer specific antigen, glypican-3 (GPC3)-targeted immunotherapy].Nihon Rinsho. 2012 Dec;70(12):2136-41. Nihon Rinsho. 2012. PMID: 23259386 Review. Japanese.
-
[Usefulness of a novel oncofetal antigen, glypican-3, for diagnosis and immunotherapy of hepatocellular carcinoma].Nihon Rinsho Meneki Gakkai Kaishi. 2008 Oct;31(5):383-91. doi: 10.2177/jsci.31.383. Nihon Rinsho Meneki Gakkai Kaishi. 2008. PMID: 18974622 Review. Japanese.
Cited by
-
Selection of highly responsive T cell receptors by an analysis combining the expression of multiple markers.Cancer Sci. 2023 Jun;114(6):2254-2264. doi: 10.1111/cas.15776. Epub 2023 Mar 28. Cancer Sci. 2023. PMID: 36866942 Free PMC article.
-
Tumor infiltrating lymphocytes as an endpoint in cancer vaccine trials.Front Immunol. 2023 Mar 7;14:1090533. doi: 10.3389/fimmu.2023.1090533. eCollection 2023. Front Immunol. 2023. PMID: 36960052 Free PMC article.
-
Immunotherapy and liver transplantation for hepatocellular carcinoma: Current and future challenges.World J Transplant. 2025 Jun 18;15(2):98509. doi: 10.5500/wjt.v15.i2.98509. World J Transplant. 2025. PMID: 40535484 Free PMC article. Review.
-
Identification of Common Cancer Antigens Useful for Specific Immunotherapies to Colorectal Cancer and Liver Metastases.Int J Mol Sci. 2025 Jul 31;26(15):7402. doi: 10.3390/ijms26157402. Int J Mol Sci. 2025. PMID: 40806530 Free PMC article.
-
HMGB1/GPC3 dual targeting vaccine induces dendritic cells-mediated CD8+T cell immune response and elicits potential therapeutic effect in hepatocellular carcinoma.iScience. 2023 Feb 4;26(3):106143. doi: 10.1016/j.isci.2023.106143. eCollection 2023 Mar 17. iScience. 2023. PMID: 36879804 Free PMC article.
References
-
- Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: Risk factors, diagnosis and treatment. Open Access Maced J Med Sci 2015; 3:732-6; PMID: 27275318; https://doi.org/10.3889/oamjms.2015.111 - DOI - PMC - PubMed
-
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009; 27:1485-91; PMID: 19224838; https://doi.org/10.1200/JCO.2008.20.7753 - DOI - PMC - PubMed
-
- Izumi N. Diagnostic and treatment algorithm of the Japanese society of hepatology: A consensus-based practice guideline. Oncology 2010; 78 Suppl 1:78-86; PMID: 20616588; https://doi.org/10.1159/000315234 - DOI - PubMed
-
- Fitzmorris P, Shoreibah M, Anand BS, Singal AK. Management of hepatocellular carcinoma. J Cancer Res Clin Oncol 2015; 141:861-76; PMID: 25158999; https://doi.org/10.1007/s00432-014-1806-0 - DOI - PMC - PubMed
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al.. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10:25-34; PMID: 19095497; https://doi.org/10.1016/S1470-2045(08)70285-7 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
