Expression of a urokinase-type plasminogen activator during tumor growth leads to angiogenesis via galanin activation in tumor-bearing mice
- PMID: 29123986
- PMCID: PMC5666387
- DOI: 10.1002/2211-5463.12318
Expression of a urokinase-type plasminogen activator during tumor growth leads to angiogenesis via galanin activation in tumor-bearing mice
Abstract
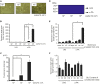
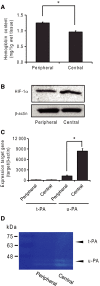
Small-cell lung carcinoma releases progalanin. The released progalanin is activated via a nonclassical processing pathway, being processed into an active form of galanin (1-20) by plasmin in extracellular components. Plasmin is produced from plasminogen activators. To clarify the regulation of progalanin via plasminogen activation by urokinase and tissue-plasminogen activator (t-PA), we investigated the regulation mechanism for urokinase and t-PA expression and their effect on galanin activation. Additionally, we studied the effect of activated galanin on angiogenesis. To determine the effect of cell density, we measured the expression levels of urokinase and t-PA using real-time PCR and plasminogen/gelatin zymography in a cell culture. The urokinase expression increased under both high cell density and presence of cell membrane fractions. However, urokinase increments induced by conditioned medium were low. These results indicate that expression of plasminogen activators is regulated by cell membrane factors. We used tumor-bearing mice to clarify the expression of plasminogen activators and galanin activation. Real-time PCR showed that urokinase was substantially higher in the central parts of tumors compared to the periphery, and this was confirmed by plasminogen/gelatin zymography. To evaluate the biological effect of plasminogen activators on tumor growth, we used tranexamic acid as a plasminogen inhibitor. Tranexamic acid decreased galanin (1-20) and the hemoglobin content of tumors and suppressed tumor growth. Additionally, galanin had no effect on the hemoglobin content of tumors derived from cells lacking GALR2. These results demonstrate the regulation of urokinase expression in tumors through progalanin activation in extracellular compartments, and confirm that galanin plays a role in angiogenesis.
Keywords: adhesion molecule; extracellular processing; galanin; neuropeptide; plasminogen activator; tumor growth.
Figures
Similar articles
-
Plasmin: its role in the extracellular processing of progalanin in tumor tissue.Protein Pept Lett. 2011 Dec;18(12):1204-11. doi: 10.2174/092986611797642751. Protein Pept Lett. 2011. PMID: 21707521
-
Involvement of plasmin-mediated extracellular activation of progalanin in angiogenesis.Biochem Biophys Res Commun. 2013 Jan 18;430(3):999-1004. doi: 10.1016/j.bbrc.2012.11.124. Epub 2012 Dec 19. Biochem Biophys Res Commun. 2013. PMID: 23261456
-
Defective processing of the transforming growth factor-beta1 in azoxymethane-induced mouse colon tumors.Mol Carcinog. 2003 May;37(1):51-9. doi: 10.1002/mc.10120. Mol Carcinog. 2003. PMID: 12720300
-
Structure, function and expression on blood and bone marrow cells of the urokinase-type plasminogen activator receptor, uPAR.Stem Cells. 1997;15(6):398-408. doi: 10.1002/stem.150398. Stem Cells. 1997. PMID: 9402652 Review.
-
The fibrinolytic system in neoplasia.Semin Thromb Hemost. 1996;22(6):459-78. doi: 10.1055/s-2007-999047. Semin Thromb Hemost. 1996. PMID: 9122711 Review.
Cited by
-
Cluster Analysis of Early Postnatal Biochemical Markers May Predict Development of Retinopathy of Prematurity.Transl Vis Sci Technol. 2020 Dec 8;9(13):14. doi: 10.1167/tvst.9.13.14. eCollection 2020 Dec. Transl Vis Sci Technol. 2020. PMID: 33344058 Free PMC article.
-
Exome sequencing in multiple sclerosis families identifies 12 candidate genes and nominates biological pathways for the genesis of disease.PLoS Genet. 2019 Jun 6;15(6):e1008180. doi: 10.1371/journal.pgen.1008180. eCollection 2019 Jun. PLoS Genet. 2019. PMID: 31170158 Free PMC article.
-
Repurposing tranexamic acid as an anticancer drug: a systematic review and meta-analysis.J Cancer Res Clin Oncol. 2025 May 9;151(5):157. doi: 10.1007/s00432-025-06185-y. J Cancer Res Clin Oncol. 2025. PMID: 40343490 Free PMC article. Review.
References
-
- Folkman J (2000) Tumor angiogenesis In Cancer Medicine (Bast RC, Jr., ed.), pp. 161–194. BC Decker, Hamilton, ON.
-
- Plate KH, Breier G, Weich HA and Risau W (1992) Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 359, 845–848. - PubMed
-
- Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD and Wiegand SJ (1999) Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284, 1994–1998. - PubMed
-
- Moscatelli D, Presta M, Joseph‐Silverstein J and Rifkin DB (1986) Both normal and tumor cells produce basic fibroblast growth factor. J Cell Physiol 129, 273–276. - PubMed
-
- Thorgeirsson UP, Lindsay CK, Cottam DW and Gomez DE (1994) Tumor invasion, proteolysis, and angiogenesis. J Neurooncol 18, 89–103. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

