E. fischeriana Root Compound Dpo Activates Antiviral Innate Immunity
- PMID: 29124042
- PMCID: PMC5662903
- DOI: 10.3389/fcimb.2017.00456
E. fischeriana Root Compound Dpo Activates Antiviral Innate Immunity
Abstract
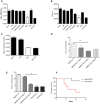
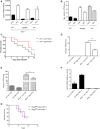
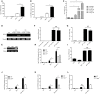
E. fischeriana has long been used as a traditional Chinese medicine. Recent studies reported that some compounds of E. fischeriana exhibited antimicrobial and immune enhance activity. Innate immune system is essential for the immune surveillance of inner and outer threats, initial host defense responses and immune modulation. The role of natural drug compounds, including E. fischeriana, in innate immune regulation is largely unknown. Here we demonstrated that E. fischeriana compound Dpo is involved in antiviral signaling. The genome wide RNA-seq analysis revealed that the induction of ISGs by viral infection could be synergized by Dpo. Consistently, Dpo enhanced the antiviral immune responses and protected the mice from death during viral infection. Dpo however was not able to rescue STING deficient mice lethality caused by HSV-1 infection. The enhancement of ISG15 by Dpo was also impaired in STING, IRF3, IRF7, or ELF4 deficient cells, demonstrating that Dpo activates innate immune responses in a STING/IRFs/ELF4 dependent way. The STING/IRFs/ELF4 axis is therefore important for Dpo induced ISGs expression, and can be used by host to counteract infection.
Keywords: RLR signalling; STING; compound; innate immunity; viruses.
Figures

References
-
- Akihisa T., KithsiriWijeratne E. M., Tokuda H., Enjo F., Toriumi M., Kimura Y., et al. (2002). Eupha-7,9(11),24-trien-3beta-ol (“antiquol C”) and other triterpenes from Euphorbia antiquorum latex and their inhibitory effects on Epstein-Barr virus activation. J. Nat. Prod. 65, 158–162. 10.1021/np010377y - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

