Membrane perturbations induced by the interactions of zinc ions with band 3 in human erythrocytes
- PMID: 29124145
- PMCID: PMC5668639
- DOI: 10.1016/j.bbrep.2015.05.003
Membrane perturbations induced by the interactions of zinc ions with band 3 in human erythrocytes
Abstract
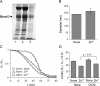
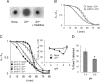
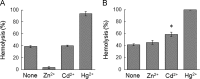
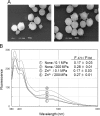
Of group 12 metals, zinc is an essential element to maintain our life, but other metals such as cadmium and mercury are toxic in cellular activities. Interactions of these metals with biomembranes are important to understand their effects on our living cells. Here, we describe the membrane perturbations induced by these metals in human erythrocytes. Of these metals, Zn2+ ions only induced the erythrocyte agglutination. Histidine residues in extracellular domains of band 3 participated in Zn2+-induced agglutination. Interestingly, it was found that band 3-cytoskeleton interactions play an important role in Zn2+-induced agglutination. In contrast with Hg2+ and Cd2+ ions, Zn2+ ions greatly suppressed pressure-induced hemolysis by cell agglutination. Such a suppression was removed upon dissociation of agglutinated erythrocytes by washing, indicating the reversible interactions of Zn2+ ions with erythrocyte membranes. Excimer fluorescence of pyrene indicated that spectrin is denatured by a pressure of 200 MPa irrespective of hemolysis suppression. Taken together, these results suggest that the agglutination of erythrocytes due to the interactions of Zn2+ ions with band 3 is stable under pressure, but spectrin, cytoskeletal protein, is denatured by pressure.
Keywords: 5P8, 5 mM sodium phosphate, pH 8; Agglutination; Band 3; C12E8, octaethylene glycol mono-n-dodecyl ether; DEPC, diethylpyrocarbonate; DIDS, 4,4′-diisothiocyanostilbene- 2,2′-disulfonate; DMPC, dimyristoylphosphatidylcholine; DNDS, 4, 4′-dinitrostilbene- 2,2′-disulfonate; Erythrocyte; Excimer; Hemolysis; NPIA, N-(1-pyrenyl) iodoacetamide; PBS, phosphate-buffered saline, 10 mM sodium phosphate, 150 mM NaCl, pH 7.4; SEM, scanning electron microscope; TBS, tris-buffered saline, 17 mM Tris, 123 mM NaCl, pH 7.4; TM, transmembrane; Zinc.
Figures


Similar articles
-
Agglutination of human erythrocytes by the interaction of Zn(2+)ion with histidine-651 on the extracellular domain of band 3.Colloids Surf B Biointerfaces. 2016 May 1;141:284-290. doi: 10.1016/j.colsurfb.2016.01.056. Epub 2016 Feb 2. Colloids Surf B Biointerfaces. 2016. PMID: 26859120
-
Membrane damages under high pressure of human erythrocytes agglutinated by concanavalin A.Colloids Surf B Biointerfaces. 2014 Apr 1;116:695-9. doi: 10.1016/j.colsurfb.2013.11.008. Epub 2013 Nov 15. Colloids Surf B Biointerfaces. 2014. PMID: 24287108
-
Effects of anion transport inhibitors on hemolysis of human erythrocytes under hydrostatic pressure.J Biochem. 1995 Oct;118(4):760-4. doi: 10.1093/oxfordjournals.jbchem.a124977. J Biochem. 1995. PMID: 8576090
-
Selenium and zinc protections against metal-(loids)-induced toxicity and disease manifestations: A review.Ecotoxicol Environ Saf. 2019 Jan 30;168:146-163. doi: 10.1016/j.ecoenv.2018.10.054. Epub 2018 Oct 29. Ecotoxicol Environ Saf. 2019. PMID: 30384162 Review.
-
Evidence for radical species as intermediates in cadmium/zinc-metallothionein-dependent DNA damage in vitro.Environ Health Perspect. 1994 Sep;102 Suppl 3(Suppl 3):27-9. doi: 10.1289/ehp.102-1567369. Environ Health Perspect. 1994. PMID: 7843112 Free PMC article. Review.
References
-
- Pomorski T.G., Nylander T., Cardenas M. Adv. Colloid Interface Sci. 2014;205:207–220. - PubMed
-
- Van Dort H.M., Moriyama R., Low P.S. J. Biol. Chem. 1998;273:14819–14826. - PubMed
-
- Yamaguchi T., Kawamura H., Kimoto E., Tanaka M. J. Biochem. 1989;106:1080–1085. - PubMed
-
- Yamaguchi T., Miyamoto J., Terada S. J. Biochem. 2001;130:597–603. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

