A single conserved basic residue in the potassium channel filter region controls KCNQ1 insensitivity toward scorpion toxins
- PMID: 29124168
- PMCID: PMC5668678
- DOI: 10.1016/j.bbrep.2015.07.003
A single conserved basic residue in the potassium channel filter region controls KCNQ1 insensitivity toward scorpion toxins
Abstract
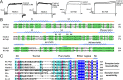
Although many studies concerning the sensitivity mechanism of scorpion toxin-potassium channel interactions have been reported, few have explored the biochemical insensitivity mechanisms of potassium channel receptors toward natural scorpion toxin peptides, such as the KCNQ1 channel. Here, by sequence alignment analyses of the human KCNQ1 channel and scorpion potassium channel MmKv2, which is completely insensitive to scorpion toxins, we proposed that the insensitivity mechanism of KCNQ1 toward natural scorpion toxins might involve two functional regions, the turret and filter regions. Based on this observation, a series of KCNQ1 mutants were constructed to study molecular mechanisms of the KCNQ1 channel insensitivity toward natural scorpion toxins. Electrophysiological studies of chimera channels showed that the channel filter region controls KCNQ1 insensitivity toward the classical scorpion toxin ChTX. Interestingly, further residue mutant experiments showed that a single basic residue in the filter region determined the insensitivity of KCNQ1 channels toward scorpion toxins. Our present work showed that amino acid residue diversification at common sites controls the sensitivity and insensitivity of potassium channels toward scorpion toxins. The unique insensitivity mechanism of KCNQ1 toward natural scorpion toxins will accelerate the rational design of potent peptide inhibitors toward this channel.
Keywords: Insensitivity; KCNQ1; Peptide design; Potassium channel; Sensitivity.
Figures



Similar articles
-
Beta-KTx14.3, a scorpion toxin, blocks the human potassium channel KCNQ1.Biochim Biophys Acta Proteins Proteom. 2023 Jul 1;1871(4):140906. doi: 10.1016/j.bbapap.2023.140906. Epub 2023 Mar 12. Biochim Biophys Acta Proteins Proteom. 2023. PMID: 36918120
-
Molecular basis for the toxin insensitivity of scorpion voltage-gated potassium channel MmKv1.Biochem J. 2016 May 1;473(9):1257-66. doi: 10.1042/BCJ20160178. Epub 2016 Mar 7. Biochem J. 2016. PMID: 26951716
-
Engineering a peptide inhibitor towards the KCNQ1/KCNE1 potassium channel (IKs).Peptides. 2015 Sep;71:77-83. doi: 10.1016/j.peptides.2015.07.002. Epub 2015 Jul 15. Peptides. 2015. PMID: 26188173
-
Diverse Structural Features of Potassium Channels Characterized by Scorpion Toxins as Molecular Probes.Molecules. 2019 May 29;24(11):2045. doi: 10.3390/molecules24112045. Molecules. 2019. PMID: 31146335 Free PMC article. Review.
-
Scorpion toxins specific for Na+-channels.Eur J Biochem. 1999 Sep;264(2):287-300. doi: 10.1046/j.1432-1327.1999.00625.x. Eur J Biochem. 1999. PMID: 10491073 Review.
Cited by
-
BmK NSPK, a Potent Potassium Channel Inhibitor from Scorpion Buthus martensii Karsch, Promotes Neurite Outgrowth via NGF/TrkA Signaling Pathway.Toxins (Basel). 2021 Jan 5;13(1):33. doi: 10.3390/toxins13010033. Toxins (Basel). 2021. PMID: 33466524 Free PMC article.
-
Extracellular modulation of TREK-2 activity with nanobodies provides insight into the mechanisms of K2P channel regulation.Nat Commun. 2024 May 16;15(1):4173. doi: 10.1038/s41467-024-48536-2. Nat Commun. 2024. PMID: 38755204 Free PMC article.
References
-
- Heil B., Ludwig J., Lichtenberg-Frate H., Lengauer T. Computational recognition of potassium channel sequences. Bioinformatics. 2006;22:1562–1568. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

