Characterization of acetohydroxyacid synthase from the hyperthermophilic bacterium Thermotoga maritima
- PMID: 29124191
- PMCID: PMC5668897
- DOI: 10.1016/j.bbrep.2015.08.014
Characterization of acetohydroxyacid synthase from the hyperthermophilic bacterium Thermotoga maritima
Abstract
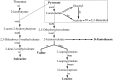
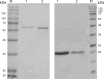
Acetohydroxyacid synthase (AHAS) is the key enzyme in branched chain amino acid biosynthesis pathway. The enzyme activity and properties of a highly thermostable AHAS from the hyperthermophilic bacterium Thermotoga maritima is being reported. The catalytic and regulatory subunits of AHAS from T. maritima were over-expressed in Escherichia coli. The recombinant subunits were purified using a simplified procedure including a heat-treatment step followed by chromatography. A discontinuous colorimetric assay method was optimized and used to determine the kinetic parameters. AHAS activity was determined to be present in several Thermotogales including T. maritima. The catalytic subunit of T. maritima AHAS was purified approximately 30-fold, with an AHAS activity of approximately 160±27 U/mg and native molecular mass of 156±6 kDa. The regulatory subunit was purified to homogeneity and showed no catalytic activity as expected. The optimum pH and temperature for AHAS activity were 7.0 and 85 °C, respectively. The apparent Km and Vmax for pyruvate were 16.4±2 mM and 246±7 U/mg, respectively. Reconstitution of the catalytic and regulatory subunits led to increased AHAS activity. This is the first report on characterization of an isoleucine, leucine, and valine operon (ilv operon) enzyme from a hyperthermophilic microorganism and may contribute to our understanding of the physiological pathways in Thermotogales. The enzyme represents the most active and thermostable AHAS reported so far.
Keywords: AHAS, acetohydroxyacid synthase; Acetohydroxyacid synthase; BCAA, branched chain amino acid; Branched-chain amino acids; CCE, crude cell extract; CFE, cell-free extract; HTCCE, heat-treated crude cell extract; Hyperthermophiles; IB, inclusion body; IMAC, immobilized metal affinity chromatography; TPP, thiamine pyrophosphate; Thermotogales; TmAHAS, Thermotoga maritima acetohydroxyacid synthase; ilv, isoleucine, leucine, valine.
Figures




Similar articles
-
Pyruvate decarboxylase activity of the acetohydroxyacid synthase of Thermotoga maritima.Biochem Biophys Rep. 2016 Jul 16;7:394-399. doi: 10.1016/j.bbrep.2016.07.008. eCollection 2016 Sep. Biochem Biophys Rep. 2016. PMID: 28955930 Free PMC article.
-
Optimization of expression and properties of the recombinant acetohydroxyacid synthase of Thermotoga maritima.Data Brief. 2015 Oct 3;5:489-97. doi: 10.1016/j.dib.2015.09.018. eCollection 2015 Dec. Data Brief. 2015. PMID: 26629492 Free PMC article.
-
Identification of the regulatory subunit of Arabidopsis thaliana acetohydroxyacid synthase and reconstitution with its catalytic subunit.Biochemistry. 2001 Jun 12;40(23):6836-44. doi: 10.1021/bi002775q. Biochemistry. 2001. PMID: 11389597
-
Acetohydroxyacid synthases: evolution, structure, and function.Appl Microbiol Biotechnol. 2016 Oct;100(20):8633-49. doi: 10.1007/s00253-016-7809-9. Epub 2016 Aug 30. Appl Microbiol Biotechnol. 2016. PMID: 27576495 Review.
-
Biosynthesis of 2-aceto-2-hydroxy acids: acetolactate synthases and acetohydroxyacid synthases.Biochim Biophys Acta. 1998 Jun 29;1385(2):401-19. doi: 10.1016/s0167-4838(98)00083-1. Biochim Biophys Acta. 1998. PMID: 9655946 Review.
Cited by
-
A thermophilic cell-free cascade enzymatic reaction for acetoin synthesis from pyruvate.Sci Rep. 2017 Jun 28;7(1):4333. doi: 10.1038/s41598-017-04684-8. Sci Rep. 2017. PMID: 28659601 Free PMC article.
-
Pyruvate decarboxylase activity of the acetohydroxyacid synthase of Thermotoga maritima.Biochem Biophys Rep. 2016 Jul 16;7:394-399. doi: 10.1016/j.bbrep.2016.07.008. eCollection 2016 Sep. Biochem Biophys Rep. 2016. PMID: 28955930 Free PMC article.
-
Preparation of a whole cell catalyst overexpressing acetohydroxyacid synthase of Thermotoga maritima and its application in the syntheses of α-hydroxyketones.Sci Rep. 2020 Sep 21;10(1):15404. doi: 10.1038/s41598-020-72416-6. Sci Rep. 2020. PMID: 32958806 Free PMC article.
-
Optimization of expression and properties of the recombinant acetohydroxyacid synthase of Thermotoga maritima.Data Brief. 2015 Oct 3;5:489-97. doi: 10.1016/j.dib.2015.09.018. eCollection 2015 Dec. Data Brief. 2015. PMID: 26629492 Free PMC article.
-
Comparative genomics of Deinococcus radiodurans: unveiling genetic discrepancies between ATCC 13939K and BAA-816 strains.Front Microbiol. 2024 Jun 19;15:1410024. doi: 10.3389/fmicb.2024.1410024. eCollection 2024. Front Microbiol. 2024. PMID: 38962131 Free PMC article.
References
-
- Stetter K. History of discovery of the first hyperthermophiles. Extremophiles. 2006;10:357–362. - PubMed
-
- Wagner I.D., Wiegel J. Diversity of thermophilic anaerobes. Ann. NY Acad. Sci. 2008;1125:1–43. - PubMed
-
- Atomi H., Sato T., Kanai T. Application of hyperthermophiles and their enzymes. Curr. Opin. Biotechnol. 2011;22:618–626. - PubMed
-
- Duggleby R.G., McCourt J.A., Guddat L.W. Structure and mechanism of inhibition of plant acetohydroxyacid synthase. Plant Physiol. Biochem. 2008;46:309–324. - PubMed
-
- Duggleby R.G., Pang S.S. Acetohydroxyacid synthase. J. Biochem. Mol. Biol. 2000;33:1–36.
LinkOut - more resources
Full Text Sources
Other Literature Sources

