1-Amino-4-hydroxy-9,10-anthraquinone - An analogue of anthracycline anticancer drugs, interacts with DNA and induces apoptosis in human MDA-MB-231 breast adinocarcinoma cells: Evaluation of structure-activity relationship using computational, spectroscopic and biochemical studies
- PMID: 29124219
- PMCID: PMC5669404
- DOI: 10.1016/j.bbrep.2015.10.008
1-Amino-4-hydroxy-9,10-anthraquinone - An analogue of anthracycline anticancer drugs, interacts with DNA and induces apoptosis in human MDA-MB-231 breast adinocarcinoma cells: Evaluation of structure-activity relationship using computational, spectroscopic and biochemical studies
Abstract
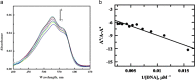
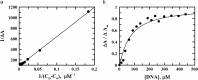
The X-ray diffraction and spectroscopic properties of 1-amino-4-hydroxy-9,10-anthraquinone (1-AHAQ), a simple analogue of anthracycline chemotherapeutic drugs were studied by adopting experimental and computational methods. The optimized geometrical parameters obtained from computational methods were compared with the results of X-ray diffraction analysis and the two were found to be in reasonably good agreement. X-ray diffraction study, Density Functional Theory (DFT) and natural bond orbital (NBO) analysis indicated two types of hydrogen bonds in the molecule. The IR spectra of 1-AHAQ were studied by Vibrational Energy Distribution Analysis (VEDA) using potential energy distribution (PED) analysis. The electronic spectra were studied by TDDFT computation and compared with the experimental results. Experimental and theoretical results corroborated each other to a fair extent. To understand the biological efficacy of 1-AHAQ, it was allowed to interact with calf thymus DNA and human breast adino-carcinoma cell MDA-MB-231. It was found that the molecule induces apoptosis in this adinocarcinoma cell, with little, if any, cytotoxic effect in HBL-100 normal breast epithelial cell.
Keywords: 1-AHAQ; 1-AHAQ, 1-amino-4-hydroxy-9,10-anthraquinone; Apoptosis; Calf thymus DNA; DFT and spectroscopy; DFT, Density Functional Theory; NBO, natural bond orbital; PED, potential energy distribution; VEDA, Vibrational Energy Distribution Analysis; X-ray diffraction; ct DNA, calf thymus DNA.
Figures







Similar articles
-
Spectroscopic, computational and electrochemical studies on the formation of the copper complex of 1-amino-4-hydroxy-9,10-anthraquinone and effect of it on superoxide formation by NADH dehydrogenase.Dalton Trans. 2015 Mar 28;44(12):5428-40. doi: 10.1039/c4dt03635b. Dalton Trans. 2015. PMID: 25691434
-
Density functional theory (DFT) and natural bond orbital (NBO) study of vibrational spectra and intramolecular hydrogen bond interaction of L-ornithine-L-aspartate.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Feb 5;136 Pt B:338-46. doi: 10.1016/j.saa.2014.08.153. Epub 2014 Oct 2. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25448937
-
Natural bond orbital analysis, electronic structure and vibrational spectral analysis of N-(4-hydroxyl phenyl) acetamide: a density functional theory.Spectrochim Acta A Mol Biomol Spectrosc. 2014 Sep 15;130:621-33. doi: 10.1016/j.saa.2014.03.065. Epub 2014 Apr 1. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24813292
-
The spectroscopic (FT-IR, UV-vis), Fukui function, NLO, NBO, NPA and tautomerism effect analysis of (E)-2-[(2-hydroxy-6-methoxybenzylidene)amino]benzonitrile.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Mar 15;139:539-48. doi: 10.1016/j.saa.2014.11.078. Epub 2014 Dec 23. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25579656
-
Vibrational spectra, molecular structure, NBO, HOMO-LUMO and first order hyperpoalarizability analysis of 1,4-bis(4-formylphenyl)anthraquinone by density functional theory.Spectrochim Acta A Mol Biomol Spectrosc. 2014 Oct 15;131:225-34. doi: 10.1016/j.saa.2014.04.085. Epub 2014 Apr 26. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24835730
Cited by
-
From In Vivo Predictive Dissolution to Virtual Bioequivalence: A GastroPlus®-Driven Framework for Generic Candesartan Cilexetil Tablets.Pharmaceuticals (Basel). 2025 Apr 11;18(4):562. doi: 10.3390/ph18040562. Pharmaceuticals (Basel). 2025. PMID: 40283997 Free PMC article.
-
Effect of Substituents on Solubility, Medicinal, Absorption, Emission and Cationic/Anionic Detection Properties of Anthraquinone Derivatives.J Fluoresc. 2024 Jul;34(4):1527-1544. doi: 10.1007/s10895-023-03410-0. Epub 2023 Aug 30. J Fluoresc. 2024. PMID: 37646872 Review.
-
An Enzymatic Oxidation Cascade Converts δ-Thiolactone Anthracene to Anthraquinone in the Biosynthesis of Anthraquinone-Fused Enediynes.JACS Au. 2024 Aug 9;4(8):2925-2935. doi: 10.1021/jacsau.4c00279. eCollection 2024 Aug 26. JACS Au. 2024. PMID: 39211597 Free PMC article.
-
Solubility of Cinnarizine in (Transcutol + Water) Mixtures: Determination, Hansen Solubility Parameters, Correlation, and Thermodynamics.Molecules. 2021 Nov 22;26(22):7052. doi: 10.3390/molecules26227052. Molecules. 2021. PMID: 34834144 Free PMC article.
-
Chrysophanol-8-O-glucoside protects mice against acute liver injury by inhibiting autophagy in hepatic stellate cells and inflammatory response in liver-resident macrophages.Front Pharmacol. 2022 Sep 6;13:951521. doi: 10.3389/fphar.2022.951521. eCollection 2022. Front Pharmacol. 2022. PMID: 36147355 Free PMC article.
References
-
- Hardman J.G., Gilman A.G., Limbird L.E. 9th ed. McGraw-Hill Companies; New York: 1996. Goodman and Gilman’s the Pharmacological Basis of Therapeutics.
-
- Hu F.Q., Liu L.N., Du Y.Z., Yuan H. Biomaterials. 2009;30:6955–6963. - PubMed
-
- Lim K.H., Kim H.S., Yang Y.M., Lee S.D., Kim W.B., Yang J., Park J.G. Cancer Chemother. Pharmacol. 1997;40:23–30. - PubMed
-
- Binaschi M., Capranico G., Dal Bo. L., Zunino F. Mol. Pharmacol. 1997;62:1053–1057. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

