Tetracyclines downregulate the production of LPS-induced cytokines and chemokines in THP-1 cells via ERK, p38, and nuclear factor-κB signaling pathways
- PMID: 29124230
- PMCID: PMC5669446
- DOI: 10.1016/j.bbrep.2015.11.003
Tetracyclines downregulate the production of LPS-induced cytokines and chemokines in THP-1 cells via ERK, p38, and nuclear factor-κB signaling pathways
Abstract
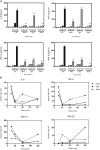
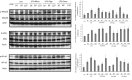
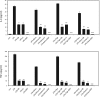
Recent reports have shown that antibiotics such as macrolide, aminoglycoside, and tetracyclines have immunomodulatory effects in addition to essential antibiotic effects. These agents may have important effects on the regulation of cytokine and chemokine production. However, the precise mechanism is unknown. This time, we used Multi Plex to measure the production of cytokines and chemokines following tetracycline treatment of lipopolysaccharide (LPS)-induced THP-1 cells. The signaling pathways were investigated with Western blotting analysis. Three tetracyclines significantly suppressed the expression of cytokines and chemokines induced by LPS. Minocycline (50 μg/ml), tigecycline (50 μg/ml), or doxycycline (50 μg/ml) were added after treatment with LPS (10 μg/ml). Tumor necrosis factor-α was downregulated to 16%, 14%, and 8%, respectively, after 60 min compared to treatment with LPS without agents. Interleukin-8 was downregulated to 43%, 32%, and 26% at 60 min. Macrophage inflammatory protein (MIP)-1α was downregulated to 23%, 33%, and 16% at 120 min. MIP-1β was downregulated to 21%, 11%, and 2% at 120 min. Concerning about signaling pathways, the mechanisms of the three tetracyclines might not be the same. Although the three tetracyclines showed some differences in the time course, tetracyclines modulated phosphorylation of the NF-κB pathway, p38 and ERK1/2/MAPK pathways, resulting in inhibition of cytokine and chemokine production. In addition, SB203580 (p38 inhibitor) and U0126 (ERK1/2 inhibitor) significantly suppressed the expression of TNF-α and IL-8 in LPS-stimulated THP-1 cells. And further, the NF-κB inhibitor, BAY11-7082, almost completely suppressed LPS-induced these two cytokines production. Thus, more than one signaling pathway may be involved in tetracyclines downregulation of the expression of LPS-induced cytokines and chemokines in THP-1 cells. And among the three signaling pathways, NF-κB pathway might be the dominant pathway on tetracyclines modification the LPS-induced cytokines production in THP-1 cells. In general, minocycline and doxycycline suppressed the production of cytokines and chemokines in LPS-stimulated THP-1 cell lines via mainly the inhibition of phosphorylation of NF-κB pathways. Tigecycline has the same structure as the other tetracyclines, however, it showed the different properties of cytokine modulation in the experimental time course.
Keywords: Chemokine; Cytokine; IKKα, inhibitor kappa B kinase alpha; IKKβ, inhibitor kappa B kinase beta; IκBα/β, inhibitor kappa B alpha and beta; LPS, lipopolysaccharide; Lipopolysaccharide (LPS); Mitogen-activated protein kinase (MAPK); NF-κB, nuclear factor-kappa B; Nuclear factor kappa B (NF-κB); Tetracyclines.
Figures

Similar articles
-
Minocycline modulates cytokine and chemokine production in lipopolysaccharide-stimulated THP-1 monocytic cells by inhibiting IκB kinase α/β phosphorylation.Transl Res. 2013 Feb;161(2):99-109. doi: 10.1016/j.trsl.2012.10.001. Epub 2012 Oct 27. Transl Res. 2013. PMID: 23108365
-
Rho-kinase inhibitor Y-27632 downregulates LPS-induced IL-6 and IL-8 production via blocking p38 MAPK and NF-κB pathways in human gingival fibroblasts.J Periodontol. 2018 Jul;89(7):883-893. doi: 10.1002/JPER.17-0571. J Periodontol. 2018. PMID: 29630729
-
Unfractionated Heparin Modulates Lipopolysaccharide-Induced Cytokine Production by Different Signaling Pathways in THP-1 Cells.J Interferon Cytokine Res. 2018 Jul;38(7):283-289. doi: 10.1089/jir.2018.0042. J Interferon Cytokine Res. 2018. PMID: 30016181
-
Effects of cytokines and chemokines on migration of mesenchymal stem cells following spinal cord injury.Neural Regen Res. 2012 May 15;7(14):1106-12. doi: 10.3969/j.issn.1673-5374.2012.14.010. Neural Regen Res. 2012. PMID: 25722702 Free PMC article. Review.
-
GOLD-Induced Cytokine (GOLDIC): A Critical Review of Its Properties, Synthesis, and Biomedical Applications.Cureus. 2024 Jan 11;16(1):e52130. doi: 10.7759/cureus.52130. eCollection 2024 Jan. Cureus. 2024. PMID: 38344607 Free PMC article. Review.
Cited by
-
Topical, systemic and biologic therapies in hidradenitis suppurativa: pathogenic insights by examining therapeutic mechanisms.Ther Adv Chronic Dis. 2019 Mar 1;10:2040622319830646. doi: 10.1177/2040622319830646. eCollection 2019. Ther Adv Chronic Dis. 2019. PMID: 30854183 Free PMC article. Review.
-
Hidradenitis suppurativa: infection, autoimmunity, or both?Ther Adv Musculoskelet Dis. 2019 Dec 30;11:1759720X19895488. doi: 10.1177/1759720X19895488. eCollection 2019. Ther Adv Musculoskelet Dis. 2019. PMID: 31908656 Free PMC article. Review.
-
Immunomodulatory Effect of Doxycycline Ameliorates Systemic and Pulmonary Inflammation in a Murine Polymicrobial Sepsis Model.Inflammation. 2020 Jun;43(3):1035-1043. doi: 10.1007/s10753-020-01188-y. Inflammation. 2020. PMID: 31955291 Free PMC article.
-
Tetracycline and viruses: a possible treatment for COVID-19?Arch Virol. 2021 Jan;166(1):1-7. doi: 10.1007/s00705-020-04860-8. Epub 2020 Nov 2. Arch Virol. 2021. PMID: 33136210 Free PMC article. Review.
-
Doxycycline reversal of amphetamine-induced mania-like behavior is related to adjusting brain monoamine abnormalities and antioxidant effects in primary hippocampal neurons.Naunyn Schmiedebergs Arch Pharmacol. 2024 Aug;397(8):6017-6035. doi: 10.1007/s00210-024-03009-7. Epub 2024 Feb 22. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38386042
References
-
- Bostanci N., Akgül B., Tsakanika V., Allaker R.P., Hughes F.J., McKay I.J. Effects of low-dose doxycycline on cytokine secretion in human monocytes stimulated with Aggregatibacter actinomycetemcomitans. Cytokine. 2011;56:656–661. - PubMed
-
- Iwasaki H., Inoue H., Mitsuke Y., Badran A., Ikegaya S., Ueda T. Doxycycline induces apoptosis by way of caspase-3 activation with inhibition of matrix metalloproteinase in human T-lymphoblastic leukemia CCRF-CEM cells. J. Lab. Clin. Med. 2002;140:382–386. - PubMed
-
- Solomon A., Rosenblatt M., Li D.Q., Liu Z., Monroy D., Ji Z., Lokeshwar B.L., Pflugfelder S.C. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2000;41:2544–2557. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

