Gain of function of TMEM16E/ANO5 scrambling activity caused by a mutation associated with gnathodiaphyseal dysplasia
- PMID: 29124309
- PMCID: PMC5897490
- DOI: 10.1007/s00018-017-2704-9
Gain of function of TMEM16E/ANO5 scrambling activity caused by a mutation associated with gnathodiaphyseal dysplasia
Abstract
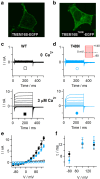
Mutations in the human TMEM16E (ANO5) gene are associated both with the bone disease gnathodiaphyseal dysplasia (GDD; OMIM: 166260) and muscle dystrophies (OMIM: 611307, 613319). However, the physiological function of TMEM16E has remained unclear. We show here that human TMEM16E, when overexpressed in mammalian cell lines, displayed partial plasma membrane localization and gave rise to phospholipid scrambling (PLS) as well as non-selective ionic currents with slow time-dependent activation at highly depolarized membrane potentials. While the activity of wild-type TMEM16E depended on elevated cytosolic Ca2+ levels, a mutant form carrying the GDD-causing T513I substitution showed PLS and large time-dependent ion currents even at low cytosolic Ca2+ concentrations. Contrarily, mutation of the homologous position in the Ca2+-activated Cl- channel TMEM16B paralog hardly affected its function. In summary, these data provide the first direct demonstration of Ca2+-dependent PLS activity for TMEM16E and suggest a gain-of-function phenotype related to a GDD mutation.
Keywords: Anoctamin5; Anoctamins; Calcium-activated chloride channels; Phosphatidylserine; Phospholipid scramblase; TMEM16E.
Conflict of interest statement
None of the authors have any competing interests.
Figures
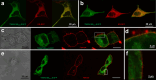


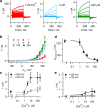
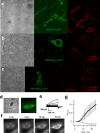
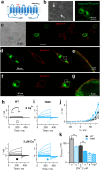
References
-
- Akasaka Y, Nakajima T, Koyama K, Furuya K, Mitsuka Y. Familial cases of a new systemic bone disease, hereditary gnatho-diaphyseal sclerosis. Nihon Seikeigeka Gakkai Zasshi. 1969;43:381–394. - PubMed
-
- Andreeva TV, Tyazhelova TV, Rykalina VN, Gusev FE, Goltsov AY, Zolotareva OI, Aliseichik MP, Borodina TA, Grigorenko AP, Reshetov DA, et al. Whole exome sequencing links dental tumor to an autosomal-dominant mutation in ANO5 gene associated with gnathodiaphyseal dysplasia and muscle dystrophies. Sci Rep. 2016;6:26440. doi: 10.1038/srep26440. - DOI - PMC - PubMed
-
- Bolduc V, Marlow G, Boycott KM, Saleki K, Inoue H, Kroon J, Itakura M, Robitaille Y, Parent L, Baas F, et al. Recessive mutations in the putative calcium-activated chloride channel Anoctamin 5 cause proximal LGMD2L and distal MMD3 muscular dystrophies. Am J Hum Genet. 2010;86:213–221. doi: 10.1016/j.ajhg.2009.12.013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

