Xeroderma Pigmentosa Group A (XPA), Nucleotide Excision Repair and Regulation by ATR in Response to Ultraviolet Irradiation
- PMID: 29124689
- PMCID: PMC6597250
- DOI: 10.1007/978-3-319-56017-5_4
Xeroderma Pigmentosa Group A (XPA), Nucleotide Excision Repair and Regulation by ATR in Response to Ultraviolet Irradiation
Abstract
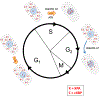
The sensitivity of Xeroderma pigmentosa (XP) patients to sunlight has spurred the discovery and genetic and biochemical analysis of the eight XP gene products (XPA-XPG plus XPV) responsible for this disorder. These studies also have served to elucidate the nucleotide excision repair (NER) process, especially the critical role played by the XPA protein. More recent studies have shown that NER also involves numerous other proteins normally employed in DNA metabolism and cell cycle regulation. Central among these is ataxia telangiectasia and Rad3-related (ATR), a protein kinase involved in intracellular signaling in response to DNA damage, especially DNA damage-induced replicative stresses. This review summarizes recent findings on the interplay between ATR as a DNA damage signaling kinase and as a novel ligand for intrinsic cell death proteins to delay damage-induced apoptosis, and on ATR's regulation of XPA and the NER process for repair of UV-induced DNA adducts. ATR's regulatory role in the cytosolic-to-nuclear translocation of XPA will be discussed. In addition, recent findings elucidating a non-NER role for XPA in DNA metabolism and genome stabilization at ds-ssDNA junctions, as exemplified in prematurely aging progeroid cells, also will be reviewed.
Keywords: ATR-XPA interaction; Ataxia telangiectasia and Rad3-related (ATR); Non-NER functions of XPA; Nucleotide excision repair (NER); Regulation of XPA by ATR; UV irradiation; XPA cytoplasmic interactions and functions; XPA nuclear import; XPA phosphorylation and acetylation; Xeroderma pigmentosum Group A (XPA).
Figures
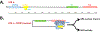
Similar articles
-
Checkpoint kinase ATR promotes nucleotide excision repair of UV-induced DNA damage via physical interaction with xeroderma pigmentosum group A.J Biol Chem. 2009 Sep 4;284(36):24213-22. doi: 10.1074/jbc.M109.000745. Epub 2009 Jul 8. J Biol Chem. 2009. PMID: 19586908 Free PMC article.
-
Phosphorylation of nucleotide excision repair factor xeroderma pigmentosum group A by ataxia telangiectasia mutated and Rad3-related-dependent checkpoint pathway promotes cell survival in response to UV irradiation.Cancer Res. 2006 Mar 15;66(6):2997-3005. doi: 10.1158/0008-5472.CAN-05-3403. Cancer Res. 2006. PMID: 16540648 Free PMC article.
-
ATR-dependent checkpoint modulates XPA nuclear import in response to UV irradiation.Oncogene. 2007 Feb 1;26(5):757-64. doi: 10.1038/sj.onc.1209828. Epub 2006 Jul 24. Oncogene. 2007. PMID: 16862173 Free PMC article.
-
Transcriptional and Posttranslational Regulation of Nucleotide Excision Repair: The Guardian of the Genome against Ultraviolet Radiation.Int J Mol Sci. 2016 Nov 4;17(11):1840. doi: 10.3390/ijms17111840. Int J Mol Sci. 2016. PMID: 27827925 Free PMC article. Review.
-
XPA: A key scaffold for human nucleotide excision repair.DNA Repair (Amst). 2016 Aug;44:123-135. doi: 10.1016/j.dnarep.2016.05.018. Epub 2016 May 20. DNA Repair (Amst). 2016. PMID: 27247238 Free PMC article. Review.
Cited by
-
Enzyme-free optical DNA mapping of the human genome using competitive binding.Nucleic Acids Res. 2019 Sep 5;47(15):e89. doi: 10.1093/nar/gkz489. Nucleic Acids Res. 2019. PMID: 31165870 Free PMC article.
-
Focus on UV-Induced DNA Damage and Repair-Disease Relevance and Protective Strategies.Int J Mol Sci. 2020 Oct 1;21(19):7264. doi: 10.3390/ijms21197264. Int J Mol Sci. 2020. PMID: 33019598 Free PMC article. Review.
-
Increased levels of XPA might be the basis of cisplatin resistance in germ cell tumours.BMC Cancer. 2020 Jan 6;20(1):17. doi: 10.1186/s12885-019-6496-1. BMC Cancer. 2020. PMID: 31906898 Free PMC article.
-
RHOAming Through the Nucleotide Excision Repair Pathway as a Mechanism of Cellular Response Against the Effects of UV Radiation.Front Cell Dev Biol. 2020 Aug 19;8:816. doi: 10.3389/fcell.2020.00816. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33015036 Free PMC article.
-
Prolyl Isomerization-Mediated Conformational Changes Define ATR Subcellular Compartment-Specific Functions.Front Cell Dev Biol. 2022 Jun 3;10:826576. doi: 10.3389/fcell.2022.826576. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35721505 Free PMC article.
References
-
- Cleaver JE, Lam ET, Revet I, and (2009) Disorders of nucleotide excision repair: the genetic and molecular basis of heterogeneity, Nat Rev Genet 10, 756–768. - PubMed
-
- Sancar A (2016) Mechanisms of DNA repair by photolyase and excision nuclease (Nobel lecture), Angewandte Chemie 55, 8502–8527. - PubMed
-
- Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, and Linn S (2004) Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints, Annu Rev Biochem 73, 39–85. - PubMed
-
- Sugasawa K (2016) Molecular mechanisms of DNA damage recognition for mammalian nucleotide excision repair, DNA Repair 44, 110–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

