Usefulness of knockout mice to clarify the role of the opioid system in chronic pain
- PMID: 29124744
- PMCID: PMC6016633
- DOI: 10.1111/bph.14088
Usefulness of knockout mice to clarify the role of the opioid system in chronic pain
Abstract
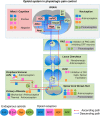
Several lines of knockout mice deficient in the genes encoding each component of the endogenous opioid system have been used for decades to clarify the specific role of the different opioid receptors and peptide precursors in many physiopathological conditions. The use of these genetically modified mice has improved our knowledge of the specific involvement of each endogenous opioid component in nociceptive transmission during acute and chronic pain conditions. The present review summarizes the recent advances obtained using these genetic tools in understanding the role of the opioid system in the pathophysiological mechanisms underlying chronic pain. Behavioural data obtained in these chronic pain models are discussed considering the peculiarities of the behavioural phenotype of each line of knockout mice. These studies have identified the crucial role of specific components of the opioid system in different manifestations of chronic pain and have also opened new possible therapeutic approaches, such as the development of opioid compounds simultaneously targeting several opioid receptors. However, several questions still remain open and require further experimental effort to be clarified. The novel genetic tools now available to manipulate specific neuronal populations and precise genome editing in mice will facilitate in a near future the elucidation of the role of each component of the endogenous opioid system in chronic pain.
Linked articles: This article is part of a themed section on Emerging Areas of Opioid Pharmacology. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.14/issuetoc.
© 2017 The British Pharmacological Society.
Figures



Similar articles
-
Predictive mechanisms linking brain opioids to chronic pain vulnerability and resilience.Br J Pharmacol. 2018 Jul;175(14):2778-2790. doi: 10.1111/bph.13840. Epub 2017 Jun 10. Br J Pharmacol. 2018. PMID: 28449262 Free PMC article. Review.
-
Emerging areas of opioid pharmacology.Br J Pharmacol. 2018 Jul;175(14):2715-2716. doi: 10.1111/bph.14343. Br J Pharmacol. 2018. PMID: 29939423 Free PMC article.
-
Therapeutic potential of opioid receptor heteromers in chronic pain and associated comorbidities.Br J Pharmacol. 2023 Apr;180(7):994-1013. doi: 10.1111/bph.15772. Epub 2022 Jan 21. Br J Pharmacol. 2023. PMID: 34883528 Review.
-
Allostery at opioid receptors: modulation with small molecule ligands.Br J Pharmacol. 2018 Jul;175(14):2846-2856. doi: 10.1111/bph.13823. Epub 2017 Jun 7. Br J Pharmacol. 2018. PMID: 28419415 Free PMC article. Review.
-
Insights into the function of opioid receptors from molecular dynamics simulations of available crystal structures.Br J Pharmacol. 2018 Jul;175(14):2834-2845. doi: 10.1111/bph.13774. Epub 2017 Apr 12. Br J Pharmacol. 2018. PMID: 28266020 Free PMC article. Review.
Cited by
-
Knock-In Mouse Models to Investigate the Functions of Opioid Receptors in vivo.Front Cell Neurosci. 2022 Jan 31;16:807549. doi: 10.3389/fncel.2022.807549. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35173584 Free PMC article. Review.
-
Mu and delta opioid receptors play opposite nociceptive and behavioural roles on nerve-injured mice.Br J Pharmacol. 2020 Mar;177(5):1187-1205. doi: 10.1111/bph.14911. Epub 2020 Feb 10. Br J Pharmacol. 2020. PMID: 31655493 Free PMC article.
-
Microglia Express Mu Opioid Receptor: Insights From Transcriptomics and Fluorescent Reporter Mice.Front Psychiatry. 2019 Jan 4;9:726. doi: 10.3389/fpsyt.2018.00726. eCollection 2018. Front Psychiatry. 2019. PMID: 30662412 Free PMC article.
-
Mu-Opioid Receptors Expressed in Glutamatergic Neurons are Essential for Morphine Withdrawal.Neurosci Bull. 2020 Oct;36(10):1095-1106. doi: 10.1007/s12264-020-00515-5. Epub 2020 May 25. Neurosci Bull. 2020. PMID: 32451910 Free PMC article.
-
Transgenic Mice for the Translational Study of Neuropathic Pain and Dystonia.Int J Mol Sci. 2022 Aug 2;23(15):8580. doi: 10.3390/ijms23158580. Int J Mol Sci. 2022. PMID: 35955710 Free PMC article. Review.
References
-
- Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE (1991). Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther 258: 299–303. - PubMed
-
- Baldock PA, Driessler F, Lin S, Wong IPL, Shi Y, Yulyaningsih E et al (2012). The endogenous opioid dynorphin is required for normal bone homeostasis in mice. Neuropeptides 46: 383–394. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

