New perspectives on telomerase RNA structure and function
- PMID: 29124890
- PMCID: PMC5815921
- DOI: 10.1002/wrna.1456
New perspectives on telomerase RNA structure and function
Abstract
Telomerase is an ancient ribonucleoprotein (RNP) that protects the ends of linear chromosomes from the loss of critical coding sequences through repetitive addition of short DNA sequences. These repeats comprise the telomere, which together with many accessory proteins, protect chromosomal ends from degradation and unwanted DNA repair. Telomerase is a unique reverse transcriptase (RT) that carries its own RNA to use as a template for repeat addition. Over decades of research, it has become clear that there are many diverse, crucial functions played by telomerase RNA beyond simply acting as a template. In this review, we highlight recent findings in three model systems: ciliates, yeast and vertebrates, that have shifted the way the field views the structural and mechanistic role(s) of RNA within the functional telomerase RNP complex. Viewed in this light, we hope to demonstrate that while telomerase RNA is just one example of the myriad functional RNA in the cell, insights into its structure and mechanism have wide-ranging impacts. WIREs RNA 2018, 9:e1456. doi: 10.1002/wrna.1456 This article is categorized under: RNA Structure and Dynamics > Influence of RNA Structure in Biological Systems RNA Structure and Dynamics > RNA Structure, Dynamics and Chemistry RNA Evolution and Genomics > RNA and Ribonucleoprotein Evolution.
© 2017 Wiley Periodicals, Inc.
Figures
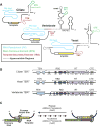
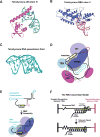


Similar articles
-
Biogenesis of telomerase ribonucleoproteins.RNA. 2012 Oct;18(10):1747-59. doi: 10.1261/rna.034629.112. Epub 2012 Aug 8. RNA. 2012. PMID: 22875809 Free PMC article. Review.
-
Evolutionary perspectives of telomerase RNA structure and function.RNA Biol. 2016 Aug 2;13(8):720-32. doi: 10.1080/15476286.2016.1205768. Epub 2016 Jun 30. RNA Biol. 2016. PMID: 27359343 Free PMC article. Review.
-
Identification of telomerase RNAs from filamentous fungi reveals conservation with vertebrates and yeasts.PLoS One. 2013;8(3):e58661. doi: 10.1371/journal.pone.0058661. Epub 2013 Mar 14. PLoS One. 2013. PMID: 23555591 Free PMC article.
-
Pseudoknot structures with conserved base triples in telomerase RNAs of ciliates.Nucleic Acids Res. 2007;35(18):6150-60. doi: 10.1093/nar/gkm660. Epub 2007 Sep 7. Nucleic Acids Res. 2007. PMID: 17827211 Free PMC article.
-
Telomerase is an unusual RNA-containing enzyme. A review.Biochemistry (Mosc). 1997 Nov;62(11):1206-15. Biochemistry (Mosc). 1997. PMID: 9467844 Review.
Cited by
-
Global Transcriptomic Analyses Reveal Genes Involved in Conceptus Development During the Implantation Stages in Pigs.Front Genet. 2021 Feb 24;12:584995. doi: 10.3389/fgene.2021.584995. eCollection 2021. Front Genet. 2021. PMID: 33719331 Free PMC article.
-
Structural Features of Nucleoprotein CST/Shelterin Complex Involved in the Telomere Maintenance and Its Association with Disease Mutations.Cells. 2020 Feb 4;9(2):359. doi: 10.3390/cells9020359. Cells. 2020. PMID: 32033110 Free PMC article. Review.
-
Crystal structures of N-terminally truncated telomerase reverse transcriptase from fungi‡.Nucleic Acids Res. 2021 May 7;49(8):4768-4781. doi: 10.1093/nar/gkab261. Nucleic Acids Res. 2021. PMID: 33856462 Free PMC article.
-
Telomerase RNAs in land plants.Nucleic Acids Res. 2019 Oct 10;47(18):9842-9856. doi: 10.1093/nar/gkz695. Nucleic Acids Res. 2019. PMID: 31392988 Free PMC article.
-
TERribly Difficult: Searching for Telomerase RNAs in Saccharomycetes.Genes (Basel). 2018 Jul 26;9(8):372. doi: 10.3390/genes9080372. Genes (Basel). 2018. PMID: 30049970 Free PMC article.
References
-
- Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. Journal of theoretical biology. 1973;41(1):181–90. - PubMed
-
- Watson JD. Origin of concatemeric T7 DNA. Nature: New biology. 1972;239(94):197–201. - PubMed
-
- Lai AG, Pouchkina-Stantcheva N, Di Donfrancesco A, Kildisiute G, Sahu S, Aboobaker AA. The protein subunit of telomerase displays patterns of dynamic evolution and conservation across different metazoan taxa. BMC evolutionary biology. 2017;17(1):107. doi: 10.1186/s12862-017-0949-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

