Lambert-Eaton myasthenic syndrome: mouse passive-transfer model illuminates disease pathology and facilitates testing therapeutic leads
- PMID: 29125190
- PMCID: PMC5790601
- DOI: 10.1111/nyas.13512
Lambert-Eaton myasthenic syndrome: mouse passive-transfer model illuminates disease pathology and facilitates testing therapeutic leads
Abstract
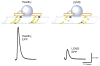
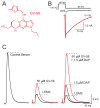
Lambert-Eaton myasthenic syndrome (LEMS) is an autoimmune disorder caused by antibodies directed against the voltage-gated calcium channels that provide the calcium ion flux that triggers acetylcholine release at the neuromuscular junction. To study the pathophysiology of LEMS and test candidate therapeutic strategies, a passive-transfer animal model has been developed in mice, which can be created by daily intraperitoneal injections of LEMS patient serum or IgG into mice for 2-4 weeks. Results from studies of the mouse neuromuscular junction have revealed that each synapse has hundreds of transmitter release sites but that the probability for release at each one is likely to be low. LEMS further reduces this low probability such that transmission is no longer effective at triggering a muscle contraction. The LEMS-mediated attack reduces the number of presynaptic calcium channels, disorganizes transmitter release sites, and results in the homeostatic upregulation of other calcium channel types. Symptomatic treatment is focused on increasing the probability of release from dysfunctional release sites. Current treatment uses the potassium channel blocker 3,4-diaminopyridine (DAP) to broaden the presynaptic action potential, providing more time for calcium channels to open. Current research is focused on testing new calcium channel gating modifiers that work synergistically with DAP.
Keywords: GV-58; Lambert-Eaton myasthenic syndrome; active zone; voltage-gated calcium channels.
© 2017 New York Academy of Sciences.
Conflict of interest statement
The authors declare no competing interests.
Figures
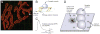


References
-
- Lambert EH, Eaton LM, Rooke ED. Defect of neuromuscular conduction associated with malignant neoplasm. Amer J Physiol. 1956;187:612–613.
-
- Vincent A, Lang B, Newsom-Davis J. Autoimmunity to the voltage-gated calcium channel underlies the Lambert-Eaton myasthenic syndrome, a paraneoplastic disorder. Trends Neurosci. 1989;12:496–502. - PubMed
-
- Nagel A, Engel AG, Lang B, et al. Lambert-Eaton myasthenic syndrome IgG depletes presynaptic membrane active zone particles by antigenic modulation. Ann Neurol. 1988;24:552–558. - PubMed
-
- Meriney SD, Hulsizer SC, Lennon VA, et al. Lambert-Eaton myasthenic syndrome IgG removes multiple types of calcium channels from a human small cell lung cancer cell line. Ann Neurol. 1996;40:739–749. - PubMed
-
- Abicht A, Lochmuller H. What’s in the serum of seronegative MG and LEMS? Neurology. 2002;59:1672–1673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

