Clinical Outcomes in Duchenne Muscular Dystrophy: A Study of 5345 Patients from the TREAT-NMD DMD Global Database
- PMID: 29125504
- PMCID: PMC5701764
- DOI: 10.3233/JND-170280
Clinical Outcomes in Duchenne Muscular Dystrophy: A Study of 5345 Patients from the TREAT-NMD DMD Global Database
Abstract
Background: Recent short-term clinical trials in patients with Duchenne Muscular Dystrophy (DMD) have indicated greater disease variability in terms of progression than expected. In addition, as average life-expectancy increases, reliable data is required on clinical progression in the older DMD population.
Objective: To determine the effects of corticosteroids on major clinical outcomes of DMD in a large multinational cohort of genetically confirmed DMD patients.
Methods: In this cross-sectional study we analysed clinical data from 5345 genetically confirmed DMD patients from 31 countries held within the TREAT-NMD global DMD database. For analysis patients were categorised by corticosteroid background and further stratified by age.
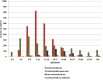
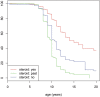
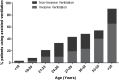
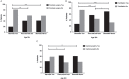
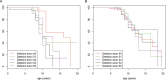
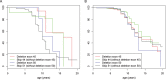
Results: Loss of ambulation in non-steroid treated patients was 10 years and in corticosteroid treated patients 13 years old (p = 0.0001). Corticosteroid treated patients were less likely to need scoliosis surgery (p < 0.001) or ventilatory support (p < 0.001) and there was a mild cardioprotective effect of corticosteroids in the patient population aged 20 years and older (p = 0.0035). Patients with a single deletion of exon 45 showed an increased survival in contrast to other single exon deletions.
Conclusions: This study provides data on clinical outcomes of DMD across many healthcare settings and including a sizeable cohort of older patients. Our data confirm the benefits of corticosteroid treatment on ambulation, need for scoliosis surgery, ventilation and, to a lesser extent, cardiomyopathy. This study underlines the importance of data collection via patient registries and the critical role of multi-centre collaboration in the rare disease field.
Keywords: DMD; Duchenne muscular dystrophy; Neuromuscular diseases; TREAT-NMD.
Conflict of interest statement
All authors declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. Professor Hanns Lochmüller was elected chair of the TREAT-NMD Alliance in April 2012. He and Jan Verschuuren both served as chairs of the oversight committee.
Figures
References
-
- Bladen CL, Rafferty K, Straub V, Monges S, Moresco A, Dawkins H, et al. The TREAT-NMD duchenne muscular dystrophy registries: Conception, design, and utilization by industry and academia. Human Mutation. 2013;34(11):1449–57. - PubMed
-
- Henricson EK, Abresch RT, Cnaan A, Hu F, Duong T, Arrieta A, et al. The cooperative international neuromuscular research group Duchenne natural history study: Glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and other commonly used clinical trial outcome measures. Muscle & Nerve. 2013;48(1):55–67. - PMC - PubMed
-
- Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R, Gastier-Foster J, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71(3):304–13. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

