Cigarette Smoke Regulates the Competitive Interactions between NRF2 and BACH1 for Heme Oxygenase-1 Induction
- PMID: 29125538
- PMCID: PMC5713355
- DOI: 10.3390/ijms18112386
Cigarette Smoke Regulates the Competitive Interactions between NRF2 and BACH1 for Heme Oxygenase-1 Induction
Abstract
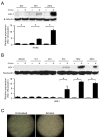
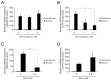
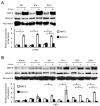
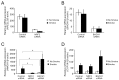
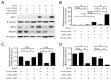
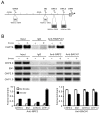
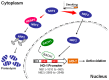
Cigarette smoke has been shown to trigger aberrant signaling pathways and pathophysiological processes; however, the regulatory mechanisms underlying smoke-induced gene expression remain to be established. Herein, we observed that two smoke-responsive genes, HO-1 and CYP1A1, are robustly induced upon smoke by different mechanisms in human bronchial epithelia. CYP1A1 is mediated by aryl hydrocarbon receptor signaling, while induction of HO-1 is regulated by oxidative stress, and suppressed by N-acetylcysteine treatment. In light of a pivotal role of NRF2 and BACH1 in response to oxidative stress and regulation of HO-1, we examined if smoke-induced HO-1 expression is modulated through the NRF2/BACH1 axis. We demonstrated that smoke causes significant nuclear translocation of NRF2, but only a slight decrease in nuclear BACH1. Knockdown of NRF2 attenuated smoke-induced HO-1 expression while down-regulation of BACH1 had stimulatory effects on both basal and smoke-induced HO-1 with trivial influence on NRF2 nuclear translocation. Chromatin immunoprecipitation assays showed that smoke augments promoter-specific DNA binding of NRF2 but suppresses BACH1 binding to the HO-1 promoter ARE sites, two of which at -1.0 kb and -2.6 kb are newly identified. These results suggest that the regulation of NRF2 activator and BACH1 repressor binding to the ARE sites are critical for smoke-mediated HO-1 induction.
Keywords: HO-1; NRF2; airway epithelium; gene regulation; smoke.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Heme oxygenase-1 induction by heat shock in rat hepatoma cell line is regulated by the coordinated function of HSF1, NRF2 and BACH1.J Biochem. 2021 Dec 4;170(4):501-510. doi: 10.1093/jb/mvab065. J Biochem. 2021. PMID: 34061198
-
Transforming growth factor-β induces transcription factors MafK and Bach1 to suppress expression of the heme oxygenase-1 gene.J Biol Chem. 2013 Jul 12;288(28):20658-67. doi: 10.1074/jbc.M113.450478. Epub 2013 Jun 4. J Biol Chem. 2013. PMID: 23737527 Free PMC article.
-
Prolonged cigarette smoke exposure decreases heme oxygenase-1 and alters Nrf2 and Bach1 expression in human macrophages: roles of the MAP kinases ERK(1/2) and JNK.FEBS Lett. 2009 Nov 3;583(21):3508-18. doi: 10.1016/j.febslet.2009.10.010. Epub 2009 Oct 12. FEBS Lett. 2009. PMID: 19822148
-
Silencing Bach1 alters aging-related changes in the expression of Nrf2-regulated genes in primary human bronchial epithelial cells.Arch Biochem Biophys. 2019 Sep 15;672:108074. doi: 10.1016/j.abb.2019.108074. Epub 2019 Aug 15. Arch Biochem Biophys. 2019. PMID: 31422075 Review.
-
CAPE and its synthetic derivative VP961 restore BACH1/NRF2 axis in Down Syndrome.Free Radic Biol Med. 2022 Apr;183:1-13. doi: 10.1016/j.freeradbiomed.2022.03.006. Epub 2022 Mar 11. Free Radic Biol Med. 2022. PMID: 35283228 Review.
Cited by
-
Neuroprotection of low dose carbon monoxide in Parkinson's disease models commensurate with the reduced risk of Parkinson's among smokers.NPJ Parkinsons Dis. 2024 Aug 22;10(1):152. doi: 10.1038/s41531-024-00763-6. NPJ Parkinsons Dis. 2024. PMID: 39174550 Free PMC article.
-
Cellular and Molecular Signatures of Oxidative Stress in Bronchial Epithelial Cell Models Injured by Cigarette Smoke Extract.Int J Mol Sci. 2022 Feb 4;23(3):1770. doi: 10.3390/ijms23031770. Int J Mol Sci. 2022. PMID: 35163691 Free PMC article. Review.
-
Multi-omics analysis identifies distinct subtypes with clinical relevance in lung adenocarcinoma harboring KEAP1/NFE2L2.J Cancer. 2022 Feb 28;13(5):1512-1522. doi: 10.7150/jca.66241. eCollection 2022. J Cancer. 2022. PMID: 35371318 Free PMC article.
-
AMPK Enhances Transcription of Selected Nrf2 Target Genes via Negative Regulation of Bach1.Front Cell Dev Biol. 2020 Jul 14;8:628. doi: 10.3389/fcell.2020.00628. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32760724 Free PMC article.
-
The Complex Genetic and Epigenetic Regulation of the Nrf2 Pathways: A Review.Antioxidants (Basel). 2023 Feb 3;12(2):366. doi: 10.3390/antiox12020366. Antioxidants (Basel). 2023. PMID: 36829925 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

